Pictet-spengler/transamination cascade reaction of indoles for modular synthesis of marinoquinoline analogues
引用次数: 0
Abstract
The Pictet-Spengler/transamination cascade reaction enables modular synthesis of marinoquinoline analogues through three-component indole ring-expansion/cyclization in the manner of novel N1–C2 cleavage of indoles. This metal-free protocol exhibits very broad functional group tolerance with up to quantitative yields. Preliminary studies on the antitumor activity of the resultant marinoquinoline analogues reveal that the indolyl-attached pyrrolo[2,3-c]quinoline product (5d) shows great potential (IC50 of 0.32 μg/mL to HeLa cells) as a promising anticancer agent in clinic.
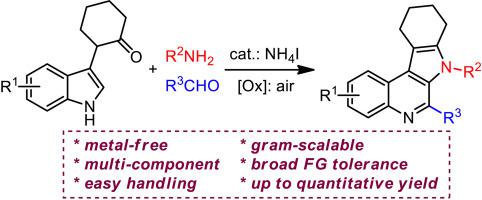
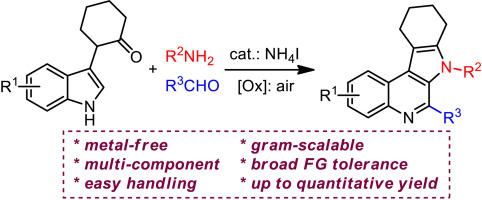
喹啉类似物模块化合成中吲哚的Pictet-spengler/转氨化级联反应
picet - spengler /转氨化级联反应以新颖的吲哚N1-C2裂解方式,通过三组分吲哚扩环/环化,模块化合成了氨基喹啉类似物。这种无金属方案具有非常广泛的官能团耐受性和定量产量。对所得的喹啉类似物抗肿瘤活性的初步研究表明,吲哚偶联吡咯[2,3-c]喹啉产物(5d)对HeLa细胞的IC50为0.32 μg/mL,具有广阔的临床应用前景。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


