Acetylene hydrogenation to ethylene by water at low temperature on a Au/α-MoC catalyst
IF 44.6
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
The hydrogenation of coal-based acetylene to ethylene is an important approach to establishing a non-oil route to ethylene production, yet it suffers from high H2 consumption as well as a high energy input. Here we report a H2-free acetylene hydrogenation process achieved by directly using water as the hydrogen source and low-cost CO as the oxygen acceptor over a Au/α-MoC catalyst. The process delivers over 99% acetylene conversion and a high ethylene selectivity of 83% at 80 °C, surpassing the hydrogenation process using H2 as the hydrogen source. Mechanistic studies have revealed that in situ-generated hydroxyl species from water dissociation at the boundary of Au and α-MoC, serving as mild reductants, enable the selective semi-hydrogenation of acetylene with residual O removed by CO. This process circumvents the need for H2 in the classical route and opens avenues for energy-efficient acetylene hydrogenation by water at low temperature. The selective hydrogenation of acetylene to ethylene involves high H2 consumption as well as a high energy input. Now, a thermocatalytic process for acetylene semi-hydrogenation using H2O as H source and CO on a Au/α-MoC catalyst is introduced.
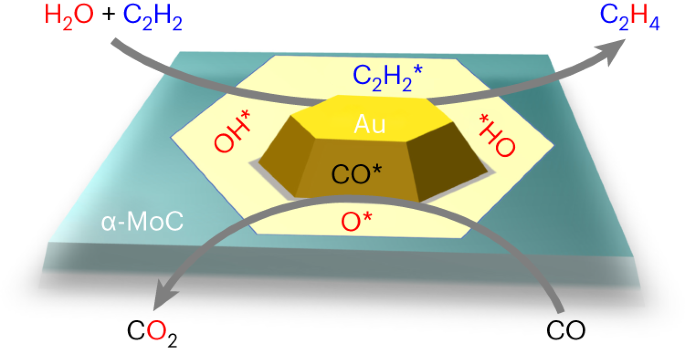
在 Au/α-MoC 催化剂上低温用水将乙炔加氢制乙烯
煤基乙炔加氢制乙烯是建立非石油乙烯生产路线的一个重要方法,但它存在高氢气消耗和高能量输入的问题。在此,我们报告了一种在 Au/α-MoC 催化剂上直接使用水作为氢源和低成本 CO 作为氧受体的无 H2 乙炔加氢工艺。该工艺的乙炔转化率超过 99%,80 °C 时的乙烯选择性高达 83%,超过了使用 H2 作为氢源的加氢工艺。机理研究表明,在金和α-MoC 的交界处水解离原位生成的羟基可作为温和的还原剂,实现乙炔的选择性半加氢,残余 O 由 CO 去除。这一过程避免了传统工艺中对 H2 的需求,为在低温下用水进行高能效乙炔氢化开辟了道路。乙炔选择性加氢制乙烯需要消耗大量的 H2 和输入高能量。现介绍一种在 Au/α-MoC 催化剂上使用 H2O 作为氢源和 CO 进行乙炔半加氢的热催化过程。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Catalysis
Chemical Engineering-Bioengineering
CiteScore
52.10
自引率
1.10%
发文量
140
期刊介绍:
Nature Catalysis serves as a platform for researchers across chemistry and related fields, focusing on homogeneous catalysis, heterogeneous catalysis, and biocatalysts, encompassing both fundamental and applied studies. With a particular emphasis on advancing sustainable industries and processes, the journal provides comprehensive coverage of catalysis research, appealing to scientists, engineers, and researchers in academia and industry.
Maintaining the high standards of the Nature brand, Nature Catalysis boasts a dedicated team of professional editors, rigorous peer-review processes, and swift publication times, ensuring editorial independence and quality. The journal publishes work spanning heterogeneous catalysis, homogeneous catalysis, and biocatalysis, covering areas such as catalytic synthesis, mechanisms, characterization, computational studies, nanoparticle catalysis, electrocatalysis, photocatalysis, environmental catalysis, asymmetric catalysis, and various forms of organocatalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


