Enantio- and Diastereoselective (Ipc)2BOTf-Mediated Aldol Reactions of Morpholine Carboxamides
IF 1.5
4区 化学
Q3 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Highly enantio- and diastereoselective (Ipc)2BOTf mediated aldol reactions of morpholine carboxamides are described. A wide variety of α-substituted N-acyl morpholine carboxamides were successfully employed, including α-bromo, α-chloro, α-vinyl and para-methoxyphenyl morpholine carboxamides which provided the corresponding aldol products in moderate to excellent yields, and generally with high enantio- and diastereoselectivities.

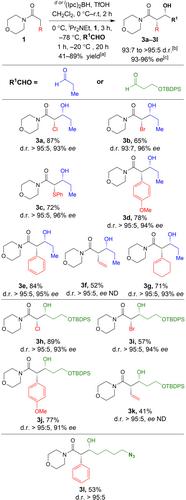
对映体和非对映体选择性 (Ipc)2BOTf 介导的吗啉羧酰胺醛醇反应
介绍了吗啉羧酰胺的高对映和非对映选择性 (Ipc)2BOTf 介导的醛醇反应。研究人员成功地使用了多种 α-取代的 N-酰基吗啉羧酰胺,包括 α-溴、α-氯、α-乙烯基和对甲氧基苯基吗啉羧酰胺,这些吗啉羧酰胺以中等到极好的产率提供了相应的醛醇产物,通常具有很高的对映和非对映选择性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Helvetica Chimica Acta
化学-化学综合
CiteScore
3.00
自引率
0.00%
发文量
60
审稿时长
2.3 months
期刊介绍:
Helvetica Chimica Acta, founded by the Swiss Chemical Society in 1917, is a monthly multidisciplinary journal dedicated to the dissemination of knowledge in all disciplines of chemistry (organic, inorganic, physical, technical, theoretical and analytical chemistry) as well as research at the interface with other sciences, where molecular aspects are key to the findings. Helvetica Chimica Acta is committed to the publication of original, high quality papers at the frontier of scientific research. All contributions will be peer reviewed with the highest possible standards and published within 3 months of receipt, with no restriction on the length of the papers and in full color.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


