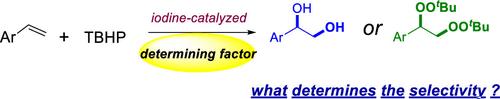
Iodine-Initiated Dioxygenation of Aryl Alkenes Using tert-Butylhydroperoxides and Water: A Route to Vicinal Diols and Bisperoxides
IF 3.3
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 4
Abstract
An environment-friendly and efficient dioxygenation of aryl alkenes for the construction of vicinal diols has been developed in water with iodine as the catalyst and tert-butylhydroperoxides (TBHPs) as the oxidant. The protocol was efficient, sustainable, and operationally simple. Detailed mechanistic studies indicated that one of the hydroxyl groups is derived from water and the other one is derived from TBHP. Additionally, the bisperoxides could be obtained in good yields with iodine as the catalyst, Na2CO3 as the additive, and propylene carbonate as the solvent, instead.
叔丁基氢过氧化物和水对芳基烯烃碘引发的双氧合反应:生成邻二醇和双过氧化物的途径
以碘为催化剂,叔丁基氢过氧化物(TBHPs)为氧化剂,在水中开发了一种环境友好、高效的芳基烯烃双氧合成邻二醇的方法。该方案高效、可持续、操作简单。详细的机理研究表明,其中一个羟基来源于水,另一个来源于三必和必拓。此外,以碘为催化剂,Na2CO3为添加剂,碳酸丙烯酯为溶剂,可以得到较好的双过氧化物。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


