下载PDF
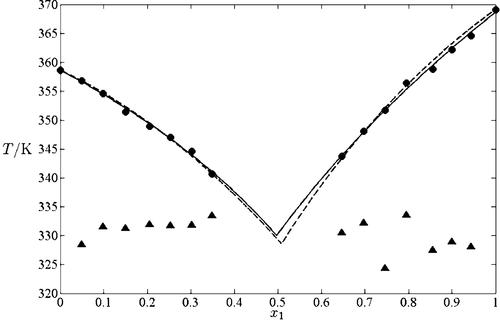
{"title":"Solid–Liquid Equilibria for Six Binary Mixtures of Pentanedioic Acid, Octanedioic Acid, 3-Methylheptanedioic Acid, 2,2-Dimethylbutanedioic Acid, and 2,3-Dimethylbutanedioic Acid","authors":"Tzu-Chi Wang*, Chia-Hao Chang","doi":"10.1021/je400686f","DOIUrl":null,"url":null,"abstract":"<p >Solid–liquid equilibria for six organic binary mixtures, namely, pentanedioic acid (1) + 3-methylheptanedioic acid (3) (eutectic temperature <i>T</i><sub>E</sub> = 329.94 K, eutectic composition <i>x</i><sub>1E</sub> = 0.497); pentanedioic acid (1) + 2,2-dimethylbutanedioic acid (4) (<i>T</i><sub>E</sub> = 354.07 K, <i>x</i><sub>1E</sub> = 0.740); pentanedioic acid (1) + 2,3-dimethylbutanedioic acid (5) (<i>T</i><sub>E</sub> = 338.95 K, <i>x</i><sub>1E</sub> = 0.415); octanedioic acid (2) + 3-methylheptanedioic acid (3) (<i>T</i><sub>E</sub> = 352.54 K, <i>x</i><sub>1E</sub> = 0.144); octanedioic acid (2) + 2,2-dimethylbutanedioic acid (4) (<i>T</i><sub>E</sub> = 386.49 K, <i>x</i><sub>1E</sub> = 0.416); and octanedioic acid (2) + 2,3-dimethylbutanedioic acid (5) (<i>T</i><sub>E</sub> = 368.30 K, <i>x</i><sub>1E</sub> = 0.193, are measured in this study using differential scanning calorimetry. Simple eutectic behaviors for these systems are observed. The experimental results are correlated using the Wilson and nonrandom two-liquid activity coefficient models, and satisfactory results are presented.</p>","PeriodicalId":42,"journal":{"name":"Journal of Chemical & Engineering Data","volume":"58 11","pages":"3233–3239"},"PeriodicalIF":2.1000,"publicationDate":"2013-10-22","publicationTypes":"Journal Article","fieldsOfStudy":null,"isOpenAccess":false,"openAccessPdf":"https://sci-hub-pdf.com/10.1021/je400686f","citationCount":"0","resultStr":null,"platform":"Semanticscholar","paperid":null,"PeriodicalName":"Journal of Chemical & Engineering Data","FirstCategoryId":"1","ListUrlMain":"https://pubs.acs.org/doi/10.1021/je400686f","RegionNum":3,"RegionCategory":"工程技术","ArticlePicture":[],"TitleCN":null,"AbstractTextCN":null,"PMCID":null,"EPubDate":"","PubModel":"","JCR":"Q3","JCRName":"CHEMISTRY, MULTIDISCIPLINARY","Score":null,"Total":0}
引用次数: 0
引用
批量引用
Abstract
Solid–liquid equilibria for six organic binary mixtures, namely, pentanedioic acid (1) + 3-methylheptanedioic acid (3) (eutectic temperature T E = 329.94 K, eutectic composition x 1E = 0.497); pentanedioic acid (1) + 2,2-dimethylbutanedioic acid (4) (T E = 354.07 K, x 1E = 0.740); pentanedioic acid (1) + 2,3-dimethylbutanedioic acid (5) (T E = 338.95 K, x 1E = 0.415); octanedioic acid (2) + 3-methylheptanedioic acid (3) (T E = 352.54 K, x 1E = 0.144); octanedioic acid (2) + 2,2-dimethylbutanedioic acid (4) (T E = 386.49 K, x 1E = 0.416); and octanedioic acid (2) + 2,3-dimethylbutanedioic acid (5) (T E = 368.30 K, x 1E = 0.193, are measured in this study using differential scanning calorimetry. Simple eutectic behaviors for these systems are observed. The experimental results are correlated using the Wilson and nonrandom two-liquid activity coefficient models, and satisfactory results are presented.
戊二酸、辛二酸、3-甲基庚二酸、2,2-二甲基丁二酸和2,3-二甲基丁二酸六种二元混合物的固液平衡
六种有机二元混合物戊二酸(1)+ 3-甲基庚二酸(3)的固液平衡(共晶温度TE = 329.94 K,共晶组成x1E = 0.497);戊二酸(1)+ 2,2-二甲基丁二酸(4)(TE = 354.07 K, x1E = 0.740);戊二酸(1)+ 2,3-二甲基丁二酸(5)(TE = 338.95 K, x1E = 0.415);辛二酸(2)+ 3-甲基庚二酸(3)(TE = 352.54 K, x1E = 0.144);辛二酸(2)+ 2,2-二甲基丁二酸(4)(TE = 386.49 K, x1E = 0.416);和辛二酸(2)+ 2,3-二甲基丁二酸(5)(TE = 368.30 K, x1E = 0.193)在本研究中采用差示扫描量热法测定。观察到这些体系的简单共晶行为。采用Wilson模型和非随机双液活度系数模型对实验结果进行了关联,得到了满意的结果。
本文章由计算机程序翻译,如有差异,请以英文原文为准。

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


