Tissue distribution and abuse potential of prucalopride: findings from non-clinical and clinical studies.
Q2 Pharmacology, Toxicology and Pharmaceutics
引用次数: 1
Abstract
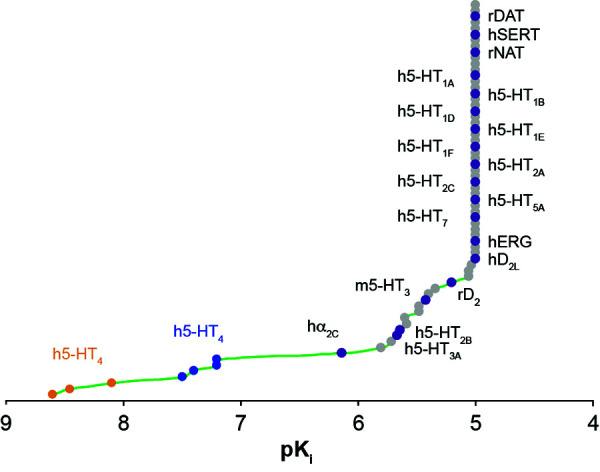
Background Prucalopride is a selective serotonin type 4 (5-HT4) receptor agonist indicated for treatment of chronic idiopathic constipation (CIC) in adults (2 mg orally, daily). 5-HT4 receptors are present in the central nervous system; therefore, non-clinical and clinical assessments were performed to evaluate the tissue distribution and abuse potential of prucalopride. Methods In vitro receptor-ligand binding studies were performed to assess the affinity of prucalopride (≤1 mM) for peptide receptors, ion channels, monoamine neurotransmitters and 5-HT receptors. The tissue distribution of 14C-prucalopride (5 mg base-equivalent/kg) was investigated in rats. Behavioural assessments in mice, rats and dogs after treatment with single or repeated (up to 24 months) subcutaneous or oral doses of prucalopride (0.02–640 mg/kg across species) were performed. Treatment-emergent adverse events possibly indicative of abuse potential during prucalopride CIC clinical trials were evaluated. Results Prucalopride showed no appreciable affinity for the receptors and ion channels investigated; its affinity (at ≤100 μM) for other 5-HT receptors was 150–10,000 times lower than that for the 5-HT4 receptor. In rats, <0.1% of the administered dose was found in the brain and concentrations were below the limit of detection within 24 hours. At supratherapeutic doses (≥20 mg/kg), mice and rats exhibited palpebral ptosis, and dogs exhibited salivation, eyelid tremors, decubitis, pedalling movements and sedation. All clinical treatment-emergent adverse events, possibly indicative of abuse potential, except dizziness, occurred in <1% of patients treated with prucalopride or placebo. Conclusion This series of non-clinical and clinical studies suggest low abuse potential for prucalopride.
普芦卡必利的组织分布和滥用潜力:来自非临床和临床研究的发现。
背景:普芦卡必利是一种选择性5-羟色胺4型(5-HT4)受体激动剂,适用于治疗成人慢性特发性便秘(CIC)(每日2毫克口服)。5-HT4受体存在于中枢神经系统;因此,通过非临床和临床评估来评估普卡必利的组织分布和滥用潜力。方法:通过体外受体-配体结合研究,评估普鲁卡普利(≤1 mM)对肽受体、离子通道、单胺类神经递质和5-HT受体的亲和力。研究14c -普芦卡必利(5 mg碱当量/kg)在大鼠体内的组织分布。对小鼠、大鼠和狗进行单次或多次(长达24个月)皮下或口服剂量(0.02-640 mg/kg,跨物种)普卡洛普利治疗后的行为评估。在普卡必利CIC临床试验期间,治疗中出现的不良事件可能指示滥用的可能性进行了评估。结果:普芦卡必利对受体和离子通道没有明显的亲和性;其对其他5-HT受体的亲和力(≤100 μM)比5-HT4受体低150 ~ 10000倍。结论:这一系列的非临床和临床研究表明普卡必利的滥用可能性很低。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Drugs in Context
Medicine-Medicine (all)
CiteScore
5.90
自引率
0.00%
发文量
63
审稿时长
9 weeks
期刊介绍:
Covers all phases of original research: laboratory, animal and human/clinical studies, health economics and outcomes research, and postmarketing studies. Original research that shows positive or negative results are welcomed. Invited review articles may cover single-drug reviews, drug class reviews, latest advances in drug therapy, therapeutic-area reviews, place-in-therapy reviews, new pathways and classes of drugs. In addition, systematic reviews and meta-analyses are welcomed and may be published as original research if performed per accepted guidelines. Editorials of key topics and issues in drugs and therapeutics are welcomed. The Editor-in-Chief will also consider manuscripts of interest in areas such as technologies that support diagnosis, assessment and treatment. EQUATOR Network reporting guidelines should be followed for each article type. GPP3 Guidelines should be followed for any industry-sponsored manuscripts. Other Editorial sections may include Editorial, Case Report, Conference Report, Letter-to-the-Editor, Educational Section.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


