Enantioselective synthesis of atropisomeric indoles via iron-catalysed oxidative cross-coupling
IF 20.2
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
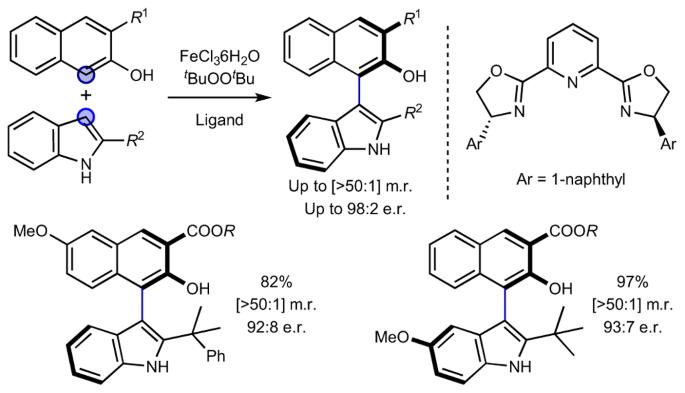
Heterobiaryl compounds that exhibit axial chirality are of increasing value and interest across several fields, but direct oxidative methods for their enantioselective synthesis remain elusive. Here we disclose that an iron catalyst in the presence of a chiral PyBOX ligand and an oxidant enables direct coupling between naphthols and indoles to yield atropisomeric heterobiaryl compounds with high levels of enantioselectivity. The reaction exhibits remarkable chemoselectivity and exclusively yields cross-coupled products without competing homocoupling. Mechanistic investigations enable us to postulate that an indole radical is generated in the reaction but that this is probably an off-cycle event, and that the reaction proceeds through formation of a chiral Fe-bound naphthoxy radical that is trapped by a nucleophilic indole. We envision that this simple, cheap and sustainable catalytic manifold will facilitate access to a range of heterobiaryl compounds and enable their application across the fields of materials science, medicinal chemistry and catalysis. Direct oxidative methods for the enantioselective synthesis of heterobiaryl compounds that exhibit axial chirality remain elusive. Now, the use of an iron catalyst in the presence of a chiral PyBOX ligand and an oxidant enables the direct coupling of naphthols and indoles with high levels of enantio- and cross-selectivity.
通过铁催化氧化交叉偶联对映体选择性合成异构吲哚
表现出轴向手性的杂二芳基化合物在多个领域具有越来越高的价值和研究兴趣,但直接氧化法对映体选择性合成杂二芳基化合物的方法仍然难以实现。在此,我们揭示了一种铁催化剂在手性 PyBOX 配体和氧化剂的存在下,能直接将萘酚和吲哚偶联,生成具有高度对映选择性的异构杂芳基化合物。该反应具有显著的化学选择性,只产生交叉偶联产物而不产生竞争性同偶联产物。机理研究使我们能够推测,反应中会产生一个吲哚自由基,但这可能是一个非周期性事件,反应是通过形成一个手性铁结合萘氧自由基进行的,该自由基被一个亲核吲哚困住。我们设想,这种简单、廉价和可持续的催化歧管将有助于获得一系列杂芳基化合物,并使其应用于材料科学、药物化学和催化等领域。直接氧化法对映选择性合成具有轴向手性的杂芳基化合物的方法仍然难以实现。现在,在存在手性 PyBOX 配体和氧化剂的情况下使用铁催化剂,可以直接偶联萘酚和吲哚,并具有很高的对映选择性和交叉选择性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature chemistry
化学-化学综合
CiteScore
29.60
自引率
1.40%
发文量
226
审稿时长
1.7 months
期刊介绍:
Nature Chemistry is a monthly journal that publishes groundbreaking and significant research in all areas of chemistry. It covers traditional subjects such as analytical, inorganic, organic, and physical chemistry, as well as a wide range of other topics including catalysis, computational and theoretical chemistry, and environmental chemistry.
The journal also features interdisciplinary research at the interface of chemistry with biology, materials science, nanotechnology, and physics. Manuscripts detailing such multidisciplinary work are encouraged, as long as the central theme pertains to chemistry.
Aside from primary research, Nature Chemistry publishes review articles, news and views, research highlights from other journals, commentaries, book reviews, correspondence, and analysis of the broader chemical landscape. It also addresses crucial issues related to education, funding, policy, intellectual property, and the societal impact of chemistry.
Nature Chemistry is dedicated to ensuring the highest standards of original research through a fair and rigorous review process. It offers authors maximum visibility for their papers, access to a broad readership, exceptional copy editing and production standards, rapid publication, and independence from academic societies and other vested interests.
Overall, Nature Chemistry aims to be the authoritative voice of the global chemical community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


