Development of fiber optic in vitro release testing method for dexamethasone release from the oil solutions.
IF 3.4
Q2 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
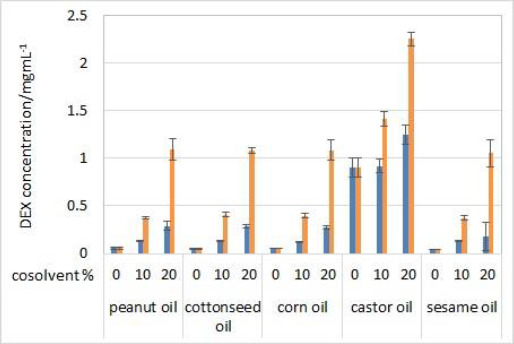
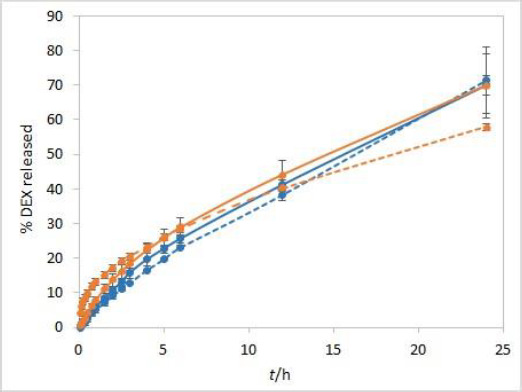
For many parenteral drugs, there is still no standardized method for in vitro release (IVR) testing available. This article presents the development of a new IVR method for oil solutions using a dialysis membrane and USP II apparatus coupled to a fiber optic UV-Vis spectrometer. Experiments were performed using dexamethasone formulations containing castor oil as a solvent with the addition of cosolvents, 20 % (v/v) of isopropanol or Capryol® 90. Based on solubility testing results, castor oil was chosen as the best solvent amongst other vegetable oils, while a significant increase in solubility was obtained by adding either of the two cosolvents. Partitioning experiments were performed to ensure these formulations could achieve prolonged drug release. IVR testing was performed with model formulations and critical test parameters were varied in order to examine the method’s sensitivity. The developed method was sensitive to temperature and stirring rate, while coupling the USP II apparatus with a fiber optic UV-Vis spectrometer enabled complete automation. Moreover, due to the interference of excipients on fiber optic detection of dexamethasone during the release testing, derivative spectroscopy was successfully introduced for the elimination of the interference. The developed IVR method described herein could be useful in preformulation investigations and the early development of novel formulations.
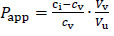
地塞米松油溶液释放度的光纤体外释放测试方法的建立。
对于许多肠外药物,仍然没有标准化的体外释放(IVR)测试方法。本文介绍了一种使用透析膜和USP II装置耦合到光纤紫外-可见光谱仪的油溶液的新IVR方法的发展。实验使用含有蓖麻油的地塞米松配方作为溶剂,并添加20% (v/v)异丙醇或Capryol®90的助溶剂。根据溶解度测试结果,在其他植物油中选择蓖麻油作为最佳溶剂,而添加两种助溶剂中的任何一种都可以显著提高溶解度。通过配药实验,确保该制剂能达到较长的释药效果。使用模型配方进行IVR测试,并改变关键测试参数,以检查该方法的灵敏度。所开发的方法对温度和搅拌速度敏感,同时将USP II仪器与光纤UV-Vis光谱仪耦合,实现了完全自动化。此外,由于辅料对地塞米松释放试验中光纤检测的干扰,我们成功地引入了导数光谱来消除干扰。本文所述开发的IVR方法可用于预制剂研究和新制剂的早期开发。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ADMET and DMPK
Multiple-
CiteScore
4.40
自引率
0.00%
发文量
22
审稿时长
4 weeks
期刊介绍:
ADMET and DMPK is an open access journal devoted to the rapid dissemination of new and original scientific results in all areas of absorption, distribution, metabolism, excretion, toxicology and pharmacokinetics of drugs. ADMET and DMPK publishes the following types of contributions: - Original research papers - Feature articles - Review articles - Short communications and Notes - Letters to Editors - Book reviews The scope of the Journal involves, but is not limited to, the following areas: - physico-chemical properties of drugs and methods of their determination - drug permeabilities - drug absorption - drug-drug, drug-protein, drug-membrane and drug-DNA interactions - chemical stability and degradations of drugs - instrumental methods in ADMET - drug metablic processes - routes of administration and excretion of drug - pharmacokinetic/pharmacodynamic study - quantitative structure activity/property relationship - ADME/PK modelling - Toxicology screening - Transporter identification and study
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


