Polycaprolactone-collagen nanofibers loaded with dexamethasone and simvastatin as an osteoinductive and immunocompatible scaffold for bone regeneration applications
Abstract
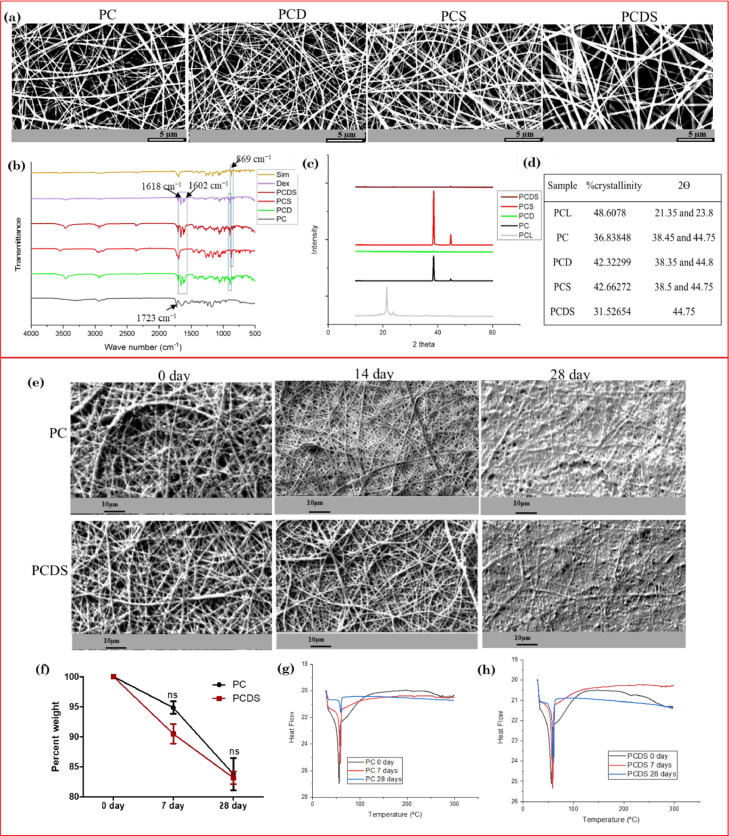
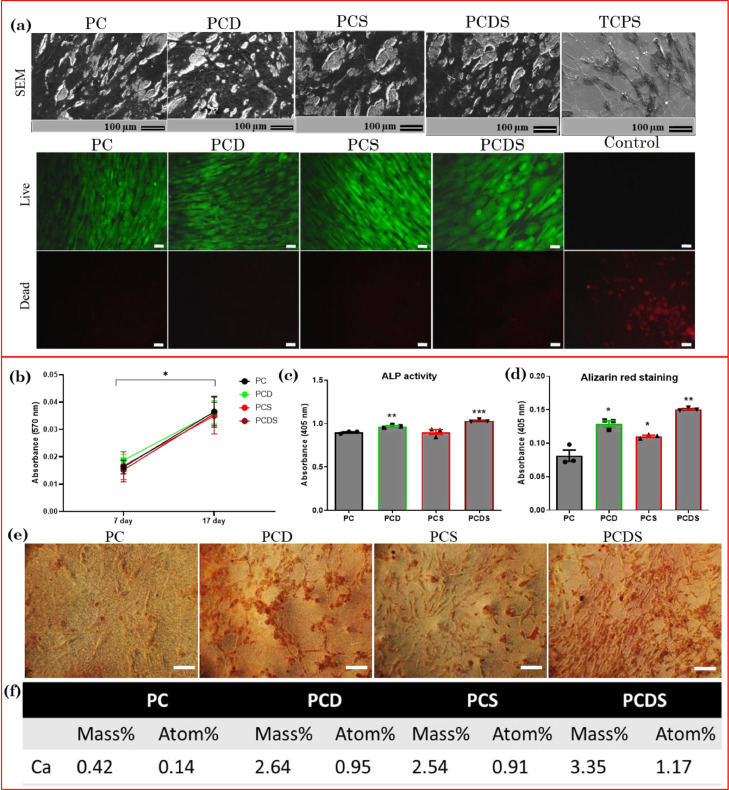
Physiological inflammation has been shown to promote bone regeneration; however, prolonged inflammation impedes the osteogenesis and bone repair process. To overcome the latter we aimed to develop a dual drug delivering nanofibrous scaffold to promote osteogenic differentiation of mesenchymal stromal cells (MSCs) and modulate the pro-inflammatory response of macrophages. The polycaprolactone (PCL)-collagen nanofibrous delivery system incorporating dexamethasone and simvastatin was fabricated by electrospinning process. The morphological analysis and mRNA, as well as protein expression of proinflammatory and anti-inflammatory cytokines in human monocytes (U937 cells), demonstrated the immunocompatibility effect of dual drug-releasing nanofibrous scaffolds. Nitric oxide estimation also demonstrated the anti-inflammatory effect of dual drug releasing scaffolds. The scaffolds demonstrated the osteogenic differentiation of adipose-derived MSCs by enhancing the alkaline phosphatase (ALP) activity and mineral deposition after 17 days of cell culture. The increased expression of Runt-related transcription factor-2 (RUNX-2) and osteocalcin at mRNA and protein levels supported the osteogenic potential of dual drug-loaded fibrous scaffolds. Hence, the results indicate that our fabricated nanofibrous scaffolds exhibit immunomodulatory properties and could be employed for bone regeneration applications after further in-vivo validation.


 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


