A membrane-based seawater electrolyser for hydrogen generation
IF 48.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 118
Abstract
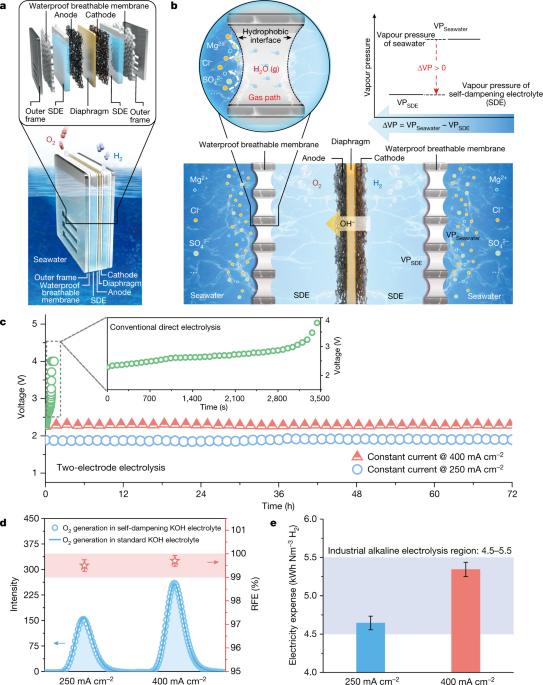
Electrochemical saline water electrolysis using renewable energy as input is a highly desirable and sustainable method for the mass production of green hydrogen1–7; however, its practical viability is seriously challenged by insufficient durability because of the electrode side reactions and corrosion issues arising from the complex components of seawater. Although catalyst engineering using polyanion coatings to suppress corrosion by chloride ions or creating highly selective electrocatalysts has been extensively exploited with modest success, it is still far from satisfactory for practical applications8–14. Indirect seawater splitting by using a pre-desalination process can avoid side-reaction and corrosion problems15–21, but it requires additional energy input, making it economically less attractive. In addition, the independent bulky desalination system makes seawater electrolysis systems less flexible in terms of size. Here we propose a direct seawater electrolysis method for hydrogen production that radically addresses the side-reaction and corrosion problems. A demonstration system was stably operated at a current density of 250 milliamperes per square centimetre for over 3,200 hours under practical application conditions without failure. This strategy realizes efficient, size-flexible and scalable direct seawater electrolysis in a way similar to freshwater splitting without a notable increase in operation cost, and has high potential for practical application. Importantly, this configuration and mechanism promises further applications in simultaneous water-based effluent treatment and resource recovery and hydrogen generation in one step. An efficient and scalable direct seawater electrolysis method for hydrogen production that addresses the side-reaction and corrosion problems associated with using seawater instead of pure water is demonstrated.
用于制氢的膜基海水电解槽
以可再生能源为输入的电化学盐水电解法是一种非常理想的、可持续的大规模生产绿色氢气的方法1-7;然而,由于电极副反应和海水复杂成分引起的腐蚀问题,其耐久性不足,使其实际可行性受到严重挑战。虽然利用聚阴离子涂层抑制氯离子腐蚀或制造高选择性电催化剂的催化剂工程已被广泛利用,并取得了一定的成功,但在实际应用中仍远远不能令人满意8-14。使用预脱盐工艺进行间接海水分离可以避免副反应和腐蚀问题15-21,但需要额外的能源投入,因此在经济上不那么有吸引力。此外,独立笨重的海水淡化系统使得海水电解系统在尺寸上不够灵活。在此,我们提出了一种直接海水电解制氢方法,从根本上解决了副反应和腐蚀问题。在实际应用条件下,一个示范系统以每平方厘米 250 毫安培的电流密度稳定运行了 3200 多个小时,没有出现故障。这一策略实现了高效、尺寸灵活和可扩展的直接海水电解,其方式与淡水分馏类似,但运行成本没有显著增加,具有很高的实际应用潜力。重要的是,这种配置和机制有望进一步应用于水基污水的同步处理、资源回收和一步制氢。展示了一种高效、可扩展的直接海水电解制氢方法,该方法解决了使用海水而非纯水所带来的副反应和腐蚀问题。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature
综合性期刊-综合性期刊
CiteScore
90.00
自引率
1.20%
发文量
3652
审稿时长
3 months
期刊介绍:
Nature is a prestigious international journal that publishes peer-reviewed research in various scientific and technological fields. The selection of articles is based on criteria such as originality, importance, interdisciplinary relevance, timeliness, accessibility, elegance, and surprising conclusions. In addition to showcasing significant scientific advances, Nature delivers rapid, authoritative, insightful news, and interpretation of current and upcoming trends impacting science, scientists, and the broader public. The journal serves a dual purpose: firstly, to promptly share noteworthy scientific advances and foster discussions among scientists, and secondly, to ensure the swift dissemination of scientific results globally, emphasizing their significance for knowledge, culture, and daily life.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


