Immunotherapies for hepatocellular carcinoma
IF 81.1
1区 医学
Q1 ONCOLOGY
引用次数: 561
Abstract
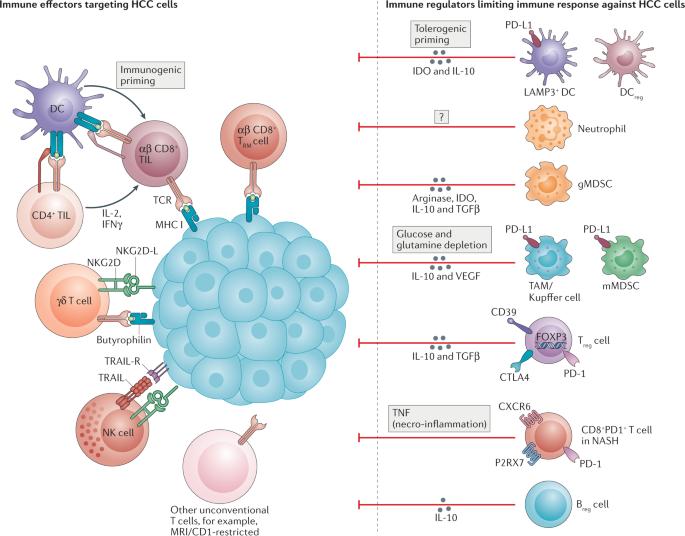
Liver cancer, more specifically hepatocellular carcinoma (HCC), is the second leading cause of cancer-related death and its incidence is increasing globally. Around 50% of patients with HCC receive systemic therapies, traditionally sorafenib or lenvatinib in the first line and regorafenib, cabozantinib or ramucirumab in the second line. In the past 5 years, immune-checkpoint inhibitors have revolutionized the management of HCC. The combination of atezolizumab and bevacizumab has been shown to improve overall survival relative to sorafenib, resulting in FDA approval of this regimen. More recently, durvalumab plus tremelimumab yielded superior overall survival versus sorafenib and atezolizumab plus cabozantinib yielded superior progression-free survival. In addition, pembrolizumab monotherapy and the combination of nivolumab plus ipilimumab have received FDA Accelerated Approval in the second-line setting based on early efficacy data. Despite these major advances, the molecular underpinnings governing immune responses and evasion remain unclear. The immune microenvironment has crucial roles in the development and progression of HCC and distinct aetiology-dependent immune features have been defined. Inflamed and non-inflamed classes of HCC and genomic signatures have been associated with response to immune-checkpoint inhibitors, yet no validated biomarker is available to guide clinical decision-making. This Review provides information on the immune microenvironments underlying the response or resistance of HCC to immunotherapies. In addition, current evidence from phase III trials on the efficacy, immune-related adverse events and aetiology-dependent mechanisms of response are described. Finally, we discuss emerging trials assessing immunotherapies across all stages of HCC that might change the management of this disease in the near future. Immunotherapy is revolutionizing the treatment of many cancers and hepatocellular carcinoma (HCC) is no exception. This Review describes the heterogeneous immune microenvironments of HCC as well as their links with the various aetiologies underlying this malignancy and with response or resistance to immunotherapies. In addition, the authors provide an overview of the current landscape of clinical trials evaluating immunotherapies across all stages of HCC.
肝细胞癌的免疫疗法
肝癌,更具体地说是肝细胞癌(HCC),是癌症相关死亡的第二大原因,其发病率在全球范围内呈上升趋势。约50%的HCC患者接受全身治疗,传统上,索拉非尼或仑伐替尼为一线治疗方案,瑞戈非尼、卡博赞替尼或拉穆单抗为二线治疗方案。在过去 5 年中,免疫检查点抑制剂彻底改变了对 HCC 的治疗。与索拉非尼相比,atezolizumab 和贝伐单抗的联合用药已被证明可改善总生存期,因此该方案获得了 FDA 批准。最近,durvalumab联合tremelimumab的总生存期优于索拉非尼,atezolizumab联合cabozantinib的无进展生存期也优于索拉非尼。此外,基于早期疗效数据,pembrolizumab 单药疗法和 nivolumab 加 ipilimumab 组合疗法在二线治疗中获得了 FDA 加速批准。尽管取得了这些重大进展,但管理免疫反应和免疫逃避的分子基础仍不清楚。免疫微环境在 HCC 的发生和发展过程中起着至关重要的作用,并且已经定义了不同的病因依赖性免疫特征。炎症和非炎症类 HCC 以及基因组特征与对免疫检查点抑制剂的反应有关,但目前尚无有效的生物标志物来指导临床决策。本综述介绍了HCC对免疫疗法的反应或耐药性所依赖的免疫微环境。此外,我们还介绍了来自 III 期试验的有关疗效、免疫相关不良事件和病因依赖性反应机制的现有证据。最后,我们讨论了新出现的评估 HCC 各个阶段免疫疗法的试验,这些试验可能会在不久的将来改变这种疾病的治疗方法。免疫疗法正在彻底改变许多癌症的治疗,肝细胞癌(HCC)也不例外。本综述描述了 HCC 的异质性免疫微环境,以及它们与这种恶性肿瘤的各种病因和对免疫疗法的反应或耐药性之间的联系。此外,作者还概述了目前评估 HCC 各个阶段免疫疗法的临床试验情况。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
99.40
自引率
0.40%
发文量
114
审稿时长
6-12 weeks
期刊介绍:
Nature Reviews publishes clinical content authored by internationally renowned clinical academics and researchers, catering to readers in the medical sciences at postgraduate levels and beyond. Although targeted at practicing doctors, researchers, and academics within specific specialties, the aim is to ensure accessibility for readers across various medical disciplines. The journal features in-depth Reviews offering authoritative and current information, contextualizing topics within the history and development of a field. Perspectives, News & Views articles, and the Research Highlights section provide topical discussions, opinions, and filtered primary research from diverse medical journals.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


