Meningeal lymphatic vessels mediate neurotropic viral drainage from the central nervous system
IF 21.2
1区 医学
Q1 NEUROSCIENCES
引用次数: 28
Abstract
Recent studies have demonstrated that brain meningeal lymphatic vessels (MLVs) act as a drainage path directly into the cervical lymph nodes (CLNs) for macromolecules contained in the cerebrospinal fluid (CSF). However, the role of MLVs during CNS viral infection remains unexplored. Here, we found that infection with several neurotropic viruses in mice promotes MLV expansion but also causes impaired MLV-mediated drainage of macromolecules. Notably, MLVs could drain virus from the CNS to CLNs. Surgical ligation of the lymph vessels or photodynamic ablation of dorsal MLVs increased neurological damage and mortality of virus-infected mice. By contrast, pretreatment with vascular endothelial growth factor C promoted expansion of functional MLVs and alleviated the effects of viral infection. Together, these data indicate that functional MLVs facilitate virus clearance, and MLVs represent a critical path for virus spreading from the CNS to the CLNs. MLV-based therapeutic strategies may thus be useful for alleviating infection-induced neurological damage. This study finds that during acute viral infection of the CNS, meningeal lymphatic vessels (MLVs) can transport virus from the CNS to draining cervical lymph nodes. VEGF-C-induced expansion of functional MLVs facilitated virus clearance.
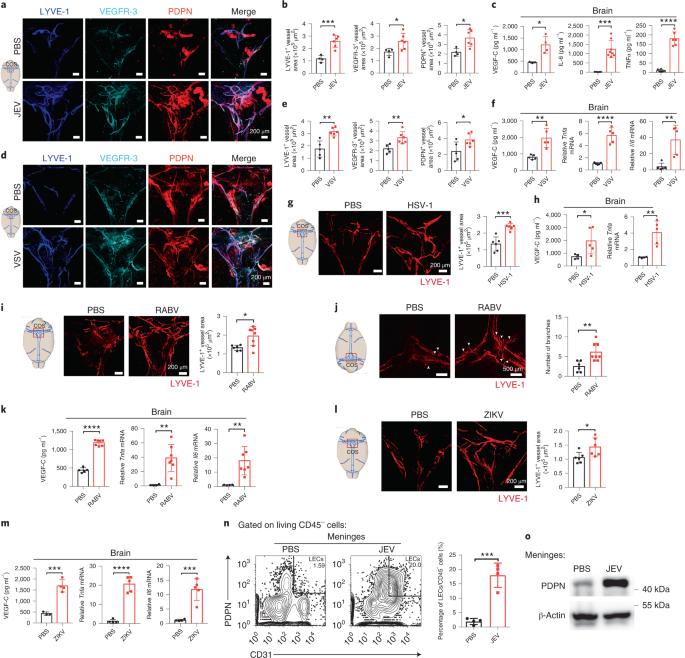
脑膜淋巴管介导神经性病毒从中枢神经系统排出
最近的研究表明,脑膜淋巴管(MLV)是脑脊液(CSF)中所含大分子物质直接进入颈淋巴结(CLN)的引流通道。然而,MLVs 在中枢神经系统病毒感染过程中的作用仍有待探索。在这里,我们发现小鼠感染几种神经毒性病毒后会促进 MLV 的扩张,但同时也会导致 MLV 介导的大分子引流受损。值得注意的是,MLV 可将病毒从中枢神经系统引流到 CLN。手术结扎淋巴管或光动力消融背侧 MLV 会增加病毒感染小鼠的神经损伤和死亡率。相比之下,使用血管内皮生长因子 C 进行预处理可促进功能性 MLV 的扩张,减轻病毒感染的影响。总之,这些数据表明,功能性 MLV 可促进病毒清除,而 MLV 是病毒从中枢神经系统扩散到 CLN 的关键路径。因此,基于 MLV 的治疗策略可能有助于减轻感染引起的神经损伤。这项研究发现,在中枢神经系统急性病毒感染期间,脑膜淋巴管(MLV)可将病毒从中枢神经系统运送到引流的颈淋巴结。VEGF-C 诱导的功能性脑膜淋巴管的扩张有助于病毒清除。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature neuroscience
医学-神经科学
CiteScore
38.60
自引率
1.20%
发文量
212
审稿时长
1 months
期刊介绍:
Nature Neuroscience, a multidisciplinary journal, publishes papers of the utmost quality and significance across all realms of neuroscience. The editors welcome contributions spanning molecular, cellular, systems, and cognitive neuroscience, along with psychophysics, computational modeling, and nervous system disorders. While no area is off-limits, studies offering fundamental insights into nervous system function receive priority.
The journal offers high visibility to both readers and authors, fostering interdisciplinary communication and accessibility to a broad audience. It maintains high standards of copy editing and production, rigorous peer review, rapid publication, and operates independently from academic societies and other vested interests.
In addition to primary research, Nature Neuroscience features news and views, reviews, editorials, commentaries, perspectives, book reviews, and correspondence, aiming to serve as the voice of the global neuroscience community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


