Different drug approaches to COVID-19 treatment worldwide: an update of new drugs and drugs repositioning to fight against the novel coronavirus.
Q2 Medicine
Therapeutic Advances in Vaccines and Immunotherapy
Pub Date : 2022-01-01
DOI:10.1177/25151355221144845
引用次数: 6
Abstract
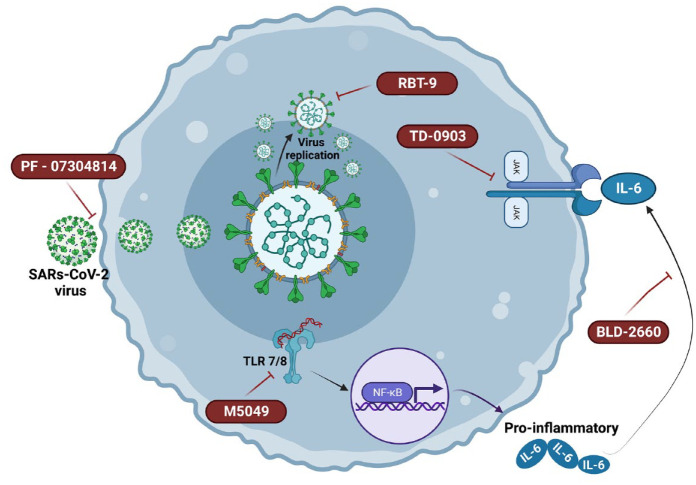
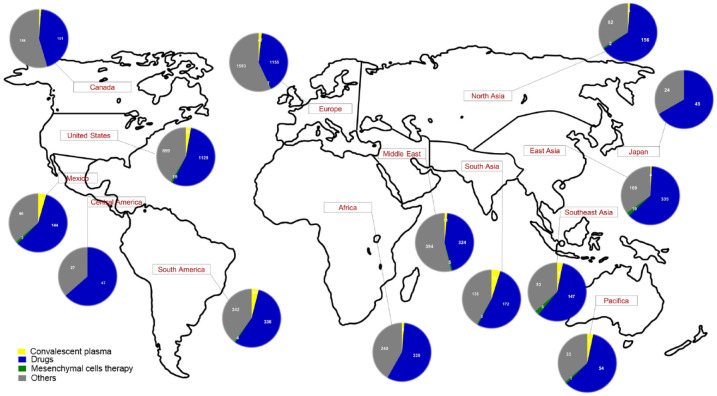
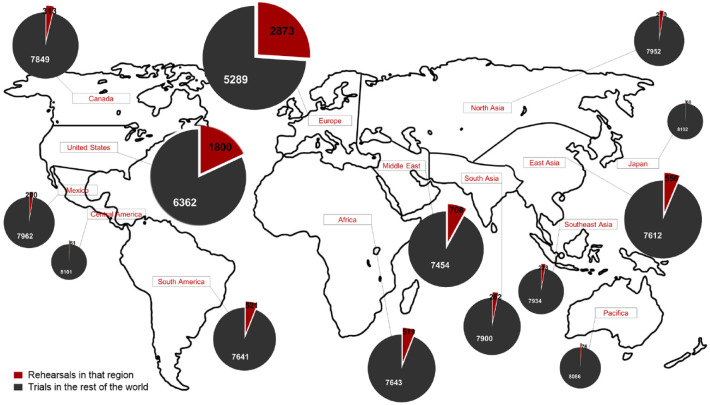
According to the World Health Organization (WHO), in the second half of 2022, there are about 606 million confirmed cases of COVID-19 and almost 6,500,000 deaths around the world. A pandemic was declared by the WHO in March 2020 when the new coronavirus spread around the world. The short time between the first cases in Wuhan and the declaration of a pandemic initiated the search for ways to stop the spread of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) or to attempt to cure the disease COVID-19. More than ever, research groups are developing vaccines, drugs, and immunobiological compounds, and they are even trying to repurpose drugs in an increasing number of clinical trials. There are great expectations regarding the vaccine’s effectiveness for the prevention of COVID-19. However, producing sufficient doses of vaccines for the entire population and SARS-CoV-2 variants are challenges for pharmaceutical industries. On the contrary, efforts have been made to create different vaccines with different approaches so that they can be used by the entire population. Here, we summarize about 8162 clinical trials, showing a greater number of drug clinical trials in Europe and the United States and less clinical trials in low-income countries. Promising results about the use of new drugs and drug repositioning, monoclonal antibodies, convalescent plasma, and mesenchymal stem cells to control viral infection/replication or the hyper-inflammatory response to the new coronavirus bring hope to treat the disease.
全球治疗COVID-19的不同药物方法:新药更新和药物重新定位以对抗新型冠状病毒
根据世界卫生组织(世卫组织)的数据,2022年下半年,全球约有6.06亿例新冠肺炎确诊病例,近650万人死亡。2020年3月,当新型冠状病毒在全球传播时,世界卫生组织宣布了一场大流行。从武汉出现第一例病例到宣布大流行之间的短暂时间,开启了寻找阻止严重急性呼吸综合征冠状病毒2 (SARS-CoV-2)传播或试图治愈COVID-19疾病的方法。研究小组比以往任何时候都更多地开发疫苗、药物和免疫生物学化合物,他们甚至试图在越来越多的临床试验中改变药物的用途。人们对该疫苗的预防效果寄予厚望。然而,为全体人口和SARS-CoV-2变体生产足够剂量的疫苗是制药行业面临的挑战。相反,已经作出努力,用不同的方法研制不同的疫苗,以便所有人都能使用。在这里,我们总结了大约8162项临床试验,欧洲和美国的药物临床试验数量较多,而低收入国家的临床试验较少。利用新药和药物重新定位、单克隆抗体、恢复期血浆和间充质干细胞控制病毒感染/复制或对新型冠状病毒的高炎症反应等方面的可喜成果为治疗该病带来了希望。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Therapeutic Advances in Vaccines and Immunotherapy
Medicine-Pharmacology (medical)
CiteScore
5.10
自引率
0.00%
发文量
15
审稿时长
8 weeks
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


