The assembly, regulation and function of the mitochondrial respiratory chain
IF 90.2
1区 生物学
Q1 CELL BIOLOGY
引用次数: 151
Abstract
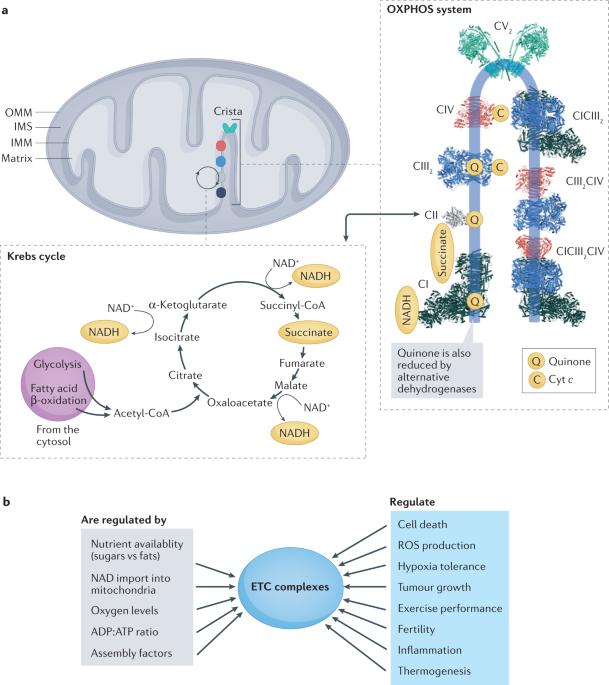
The mitochondrial oxidative phosphorylation system is central to cellular metabolism. It comprises five enzymatic complexes and two mobile electron carriers that work in a mitochondrial respiratory chain. By coupling the oxidation of reducing equivalents coming into mitochondria to the generation and subsequent dissipation of a proton gradient across the inner mitochondrial membrane, this electron transport chain drives the production of ATP, which is then used as a primary energy carrier in virtually all cellular processes. Minimal perturbations of the respiratory chain activity are linked to diseases; therefore, it is necessary to understand how these complexes are assembled and regulated and how they function. In this Review, we outline the latest assembly models for each individual complex, and we also highlight the recent discoveries indicating that the formation of larger assemblies, known as respiratory supercomplexes, originates from the association of the intermediates of individual complexes. We then discuss how recent cryo-electron microscopy structures have been key to answering open questions on the function of the electron transport chain in mitochondrial respiration and how supercomplexes and other factors, including metabolites, can regulate the activity of the single complexes. When relevant, we discuss how these mechanisms contribute to physiology and outline their deregulation in human diseases. Mitochondrial respiration relies on five enzymatic complexes that couple electron transport with proton pumping, leading to ATP synthesis. Recent studies have shed new light on the organization, assembly and mechanisms of the respiratory complexes, including the formation of their larger assemblies — respiratory supercomplexes — and their roles in physiology.
线粒体呼吸链的组装、调节和功能
线粒体氧化磷酸化系统是细胞代谢的核心。它由五个酶复合物和两个移动电子载体组成,在线粒体呼吸链中工作。通过将进入线粒体的还原等价物的氧化作用与线粒体内膜上质子梯度的产生和随后的消散耦合在一起,电子传递链驱动 ATP 的产生,然后在几乎所有细胞过程中将其用作主要的能量载体。呼吸链活动的微小干扰都与疾病有关;因此,有必要了解这些复合物是如何组装和调节的,以及它们是如何发挥作用的。在这篇综述中,我们概述了每个复合物的最新组装模型,还重点介绍了最近的发现,即更大的组装体(称为呼吸超级复合物)的形成源于单个复合物中间产物的结合。然后,我们将讨论最近的低温电子显微镜结构如何成为解答线粒体呼吸中电子传递链功能的关键,以及超级复合物和其他因素(包括代谢物)如何调控单个复合物的活性。在相关情况下,我们会讨论这些机制如何对生理学做出贡献,并概述它们在人类疾病中的失调情况。线粒体呼吸依赖于五种酶复合物,它们将电子传递与质子泵结合起来,导致 ATP 合成。最近的研究揭示了呼吸复合体的组织、组装和机制,包括它们的更大组合--呼吸超级复合体--的形成及其在生理中的作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
173.60
自引率
0.50%
发文量
118
审稿时长
6-12 weeks
期刊介绍:
Nature Reviews Molecular Cell Biology is a prestigious journal that aims to be the primary source of reviews and commentaries for the scientific communities it serves. The journal strives to publish articles that are authoritative, accessible, and enriched with easily understandable figures, tables, and other display items. The goal is to provide an unparalleled service to authors, referees, and readers, and the journal works diligently to maximize the usefulness and impact of each article. Nature Reviews Molecular Cell Biology publishes a variety of article types, including Reviews, Perspectives, Comments, and Research Highlights, all of which are relevant to molecular and cell biologists. The journal's broad scope ensures that the articles it publishes reach the widest possible audience.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


