Recent advances in the structural biology of encapsulin bacterial nanocompartments
IF 5.1
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 3
Abstract
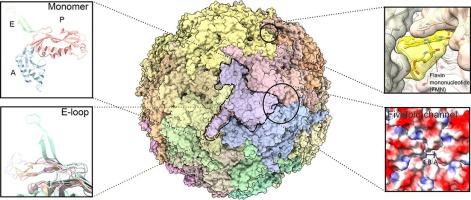
Large capsid-like nanocompartments called encapsulins are common in bacteria and archaea and contain cargo proteins with diverse functions. Advances in cryo-electron microscopy have enabled structure determination of many encapsulins in recent years. Here we summarize findings from recent encapsulin structures that have significant implications for their biological roles. We also compare important features such as the E-loop, cargo-peptide binding site, and the fivefold axis channel in different structures. In addition, we describe the discovery of a flavin-binding pocket within the encapsulin shell that may reveal a role for this nanocompartment in iron metabolism.
细菌纳米室的结构生物学研究进展
被称为胶囊的大型衣壳状纳米隔室在细菌和古细菌中很常见,含有多种功能的货物蛋白质。近年来,冷冻电镜技术的进步使许多胶囊的结构测定成为可能。在这里,我们总结了最近对其生物学作用具有重要意义的包封结构的发现。我们还比较了不同结构中的重要特征,如E-loop、货物肽结合位点和五重轴通道。此外,我们描述了在胶囊壳内发现的黄素结合袋,这可能揭示了这种纳米室在铁代谢中的作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Structural Biology: X
Biochemistry, Genetics and Molecular Biology-Structural Biology
CiteScore
6.50
自引率
0.00%
发文量
20
审稿时长
62 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


