Transcription factor Dp-1 knockdown downregulates thymidine kinase 1 expression to protect against proliferation and epithelial-mesenchymal transition in cervical cancer
Abstract
Abstract
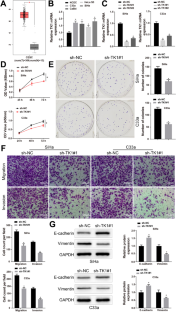
Thymidine kinase 1 (TK1) level is an independent survival prognostic factor for both premalignant and malignant cervical pathologies. Herein, this study sought to probe the impacts of TK1 on cervical cancer (CC) progression and its underlying mechanism. Transcription factor Dp-1 (TFDP1) and TK1 expression was assessed using qRT-PCR in CC cell lines. After ectopic expression and knockdown experiments, cell counting kit-8 and colony formation assays were adopted to measure cell proliferation, western blot to examine the expression of epithelial-mesenchymal transition (EMT)-related proteins, and Transwell assays to assess cell invasion and migration. The binding of TFDP1 to TK1 was predicted by bioinformatic sites and verified by chromatin immunoprecipitation and dual-luciferase reporter assays. Tumor xenograft experiments in nude mice were performed to validate the influence of TFDP1/TK1 on CC progression in vivo. CC cells had high TK1 and TFDP1 expression. TFDP1 or TK1 knockdown restrained CC cell EMT, invasion, migration, and proliferation. TFDP1 facilitated TK1 expression in CC via transcription. Overexpression of TK1 counteracted the suppressive impacts of TFDP1 knockdown on CC cell malignant behaviors. Moreover, TFDP1 knockdown depressed CC growth in vivo by downregulating TK1. TFDP1 knockdown restricted proliferation and EMT in CC by downregulating TK1 expression.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


