Enhanced tumour penetration and prolonged circulation in blood of polyzwitterion–drug conjugates with cell-membrane affinity
IF 26.8
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 88
Abstract
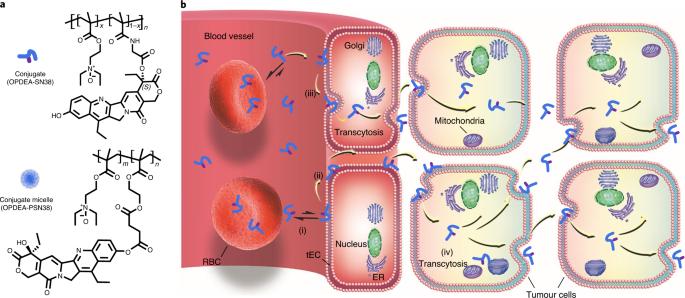
Effective anticancer nanomedicines need to exhibit prolonged circulation in blood, to extravasate and accumulate in tumours, and to be taken up by tumour cells. These contrasting criteria for persistent circulation and cell-membrane affinity have often led to complex nanoparticle designs with hampered clinical translatability. Here, we show that conjugates of small-molecule anticancer drugs with the polyzwitterion poly(2-(N-oxide-N,N-diethylamino)ethyl methacrylate) have long blood-circulation half-lives and bind reversibly to cell membranes, owing to the negligible interaction of the polyzwitterion with proteins and its weak interaction with phospholipids. Adsorption of the polyzwitterion–drug conjugates to tumour endothelial cells and then to cancer cells favoured their transcytosis-mediated extravasation into tumour interstitium and infiltration into tumours, and led to the eradication of large tumours and patient-derived tumour xenografts in mice. The simplicity and potency of the polyzwitterion–drug conjugates should facilitate the design of translational anticancer nanomedicines. Conjugates of small-molecule anticancer drugs with a polyzwitterion that has negligible interaction with proteins and a weak interaction with phospholipids eradicate large tumours and patient-derived tumour xenografts in mice.
具有细胞膜亲和力的多聚维他命-药物共轭物可增强肿瘤穿透力并延长血液循环时间
有效的抗癌纳米药物需要在血液中长期循环、外渗并在肿瘤中积聚以及被肿瘤细胞吸收。持续循环和细胞膜亲和性这两个截然不同的标准往往导致复杂的纳米粒子设计阻碍了临床转化。在这里,我们展示了小分子抗癌药物与聚齐聚(2-(N-氧化物-N,N-二乙氨基)乙基甲基丙烯酸酯)的共轭物具有较长的血液循环半衰期,并可逆地与细胞膜结合,这是因为聚齐聚与蛋白质的相互作用可忽略不计,而与磷脂的相互作用较弱。多聚维他命-药物共轭物吸附在肿瘤内皮细胞上,然后再吸附在癌细胞上,这有利于它们通过细胞外渗作用进入肿瘤间质并浸润肿瘤,从而根除小鼠体内的大肿瘤和患者衍生的肿瘤异种移植物。多聚维他命-药物共轭物的简易性和效力应有助于设计转化型抗癌纳米药物。小分子抗癌药物与聚齐聚醚的共轭物与蛋白质的相互作用可忽略不计,而与磷脂的相互作用较弱,这种共轭物能根除小鼠体内的大型肿瘤和病人来源的肿瘤异种移植物。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Biomedical Engineering
Medicine-Medicine (miscellaneous)
CiteScore
45.30
自引率
1.10%
发文量
138
期刊介绍:
Nature Biomedical Engineering is an online-only monthly journal that was launched in January 2017. It aims to publish original research, reviews, and commentary focusing on applied biomedicine and health technology. The journal targets a diverse audience, including life scientists who are involved in developing experimental or computational systems and methods to enhance our understanding of human physiology. It also covers biomedical researchers and engineers who are engaged in designing or optimizing therapies, assays, devices, or procedures for diagnosing or treating diseases. Additionally, clinicians, who make use of research outputs to evaluate patient health or administer therapy in various clinical settings and healthcare contexts, are also part of the target audience.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


