Brown-fat-mediated tumour suppression by cold-altered global metabolism
IF 50.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 53
Abstract
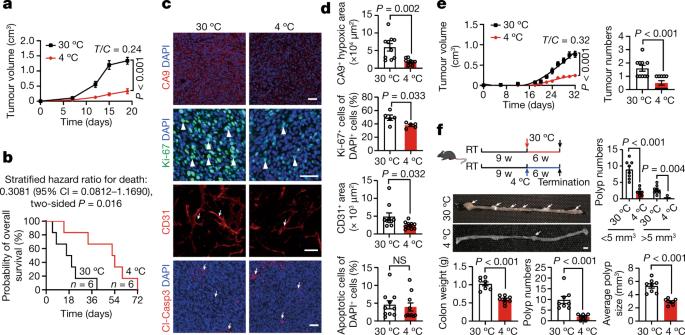
Glucose uptake is essential for cancer glycolysis and is involved in non-shivering thermogenesis of adipose tissues1–6. Most cancers use glycolysis to harness energy for their infinite growth, invasion and metastasis2,7,8. Activation of thermogenic metabolism in brown adipose tissue (BAT) by cold and drugs instigates blood glucose uptake in adipocytes4,5,9. However, the functional effects of the global metabolic changes associated with BAT activation on tumour growth are unclear. Here we show that exposure of tumour-bearing mice to cold conditions markedly inhibits the growth of various types of solid tumours, including clinically untreatable cancers such as pancreatic cancers. Mechanistically, cold-induced BAT activation substantially decreases blood glucose and impedes the glycolysis-based metabolism in cancer cells. The removal of BAT and feeding on a high-glucose diet under cold exposure restore tumour growth, and genetic deletion of Ucp1—the key mediator for BAT-thermogenesis—ablates the cold-triggered anticancer effect. In a pilot human study, mild cold exposure activates a substantial amount of BAT in both healthy humans and a patient with cancer with mitigated glucose uptake in the tumour tissue. These findings provide a previously undescribed concept and paradigm for cancer therapy that uses a simple and effective approach. We anticipate that cold exposure and activation of BAT through any other approach, such as drugs and devices either alone or in combination with other anticancer therapeutics, will provide a general approach for the effective treatment of various cancers. Mild cold exposure activates a substantial amount of brown adipose tissue (BAT) in a patient with cancer, reducing tumour-associated glucose uptake, and activation of BAT in mice inhibits the growth of tumours by decreasing blood glucose and impeding glycolysis-based metabolism in cancer cells.
寒冷改变全球新陈代谢,褐脂介导肿瘤抑制作用
葡萄糖摄取对癌症糖酵解至关重要,并参与脂肪组织的非颤抖性产热1-6。大多数癌症都利用糖酵解来为其无限生长、侵袭和转移提供能量2,7,8。寒冷和药物激活了棕色脂肪组织(BAT)的产热代谢,促使脂肪细胞摄取血糖4,5,9。然而,与棕色脂肪组织激活相关的整体代谢变化对肿瘤生长的功能性影响尚不清楚。在这里,我们发现,将罹患肿瘤的小鼠置于寒冷条件下可明显抑制各种类型实体瘤的生长,包括临床上无法治疗的癌症,如胰腺癌。从机理上讲,寒冷诱导的 BAT 激活会大幅降低血糖,阻碍癌细胞中以糖酵解为基础的新陈代谢。移除 BAT 并在低温条件下摄入高糖饮食可恢复肿瘤生长,而遗传性缺失 Ucp1(BAT 热发生的关键介质)会削弱低温触发的抗癌效果。在一项试验性人体研究中,轻度寒冷暴露激活了健康人和一名癌症患者体内大量的 BAT,同时减轻了肿瘤组织对葡萄糖的吸收。这些发现为癌症治疗提供了一个以前未曾描述过的概念和范例,它采用了一种简单而有效的方法。我们预计,通过任何其他方法(如单独使用药物和设备或与其他抗癌疗法相结合)进行冷暴露和激活 BAT,将为有效治疗各种癌症提供一种通用方法。轻度冷暴露可激活癌症患者体内大量的棕色脂肪组织(BAT),减少肿瘤相关的葡萄糖摄取,而激活小鼠体内的 BAT 可通过降低血糖和阻碍癌细胞中基于糖酵解的新陈代谢来抑制肿瘤的生长。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature
综合性期刊-综合性期刊
CiteScore
90.00
自引率
1.20%
发文量
3652
审稿时长
3 months
期刊介绍:
Nature is a prestigious international journal that publishes peer-reviewed research in various scientific and technological fields. The selection of articles is based on criteria such as originality, importance, interdisciplinary relevance, timeliness, accessibility, elegance, and surprising conclusions. In addition to showcasing significant scientific advances, Nature delivers rapid, authoritative, insightful news, and interpretation of current and upcoming trends impacting science, scientists, and the broader public. The journal serves a dual purpose: firstly, to promptly share noteworthy scientific advances and foster discussions among scientists, and secondly, to ensure the swift dissemination of scientific results globally, emphasizing their significance for knowledge, culture, and daily life.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


