An anti-CD98 antibody displaying pH-dependent Fc-mediated tumour-specific activity against multiple cancers in CD98-humanized mice
IF 26.8
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 4
Abstract
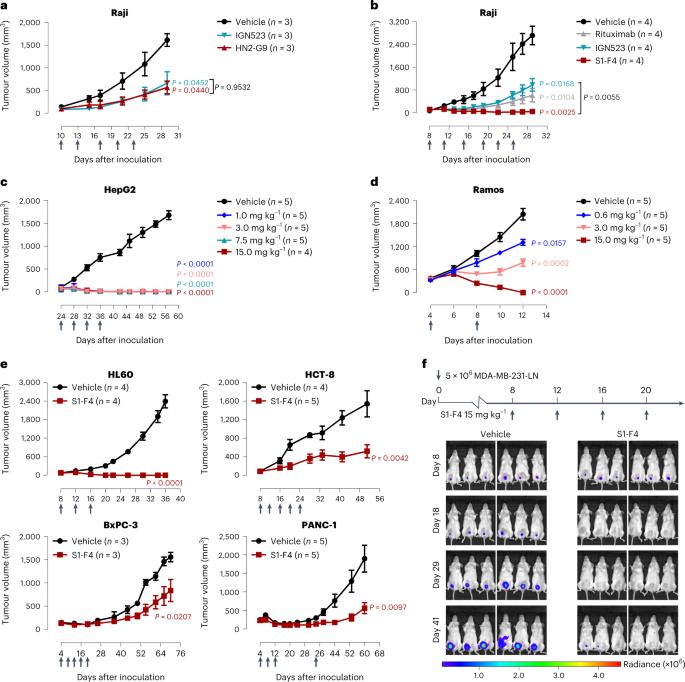
The cell-surface glycoprotein CD98—a subunit of the LAT1/CD98 amino acid transporter—is an attractive target for cancer immunotherapies, but its widespread expression has hampered the development of CD98-targeting antibody therapeutics. Here we report that an anti-CD98 antibody, identified via the screening of phage-display libraries of CD98 single-chain variable fragments with mutated complementarity-determining regions, preserves the physiological function of CD98 and elicits broad-spectrum crystallizable-fragment (Fc)-mediated anti-tumour activity (requiring Fcγ receptors for immunoglobulins, macrophages, dendritic cells and CD8+ T cells, as well as other components of the innate and adaptive immune systems) in multiple xenograft and syngeneic tumour models established in CD98-humanized mice. We also show that a variant of the anti-CD98 antibody with pH-dependent binding, generated by solving the structure of the antibody–CD98 complex, displayed enhanced tumour-specific activity and pharmacokinetics. pH-dependent antibody variants targeting widely expressed antigens may lead to superior therapeutic outcomes. An anti-CD98 antibody with pH-dependent binding elicits tumour-specific Fc-mediated anti-tumour activity in multiple xenograft and syngeneic tumour models established in CD98-humanized mice.
一种抗 CD98 抗体,在 CD98 人源化小鼠体内对多种癌症显示出 pH 依赖性 Fc 介导的肿瘤特异性活性
细胞表面糖蛋白 CD98--LAT1/CD98 氨基酸转运体的一个亚基--是癌症免疫疗法的一个诱人靶点,但它的广泛表达阻碍了 CD98 靶向抗体疗法的开发。在这里,我们报告了一种抗 CD98 抗体,该抗体是通过筛选具有突变互补决定区的 CD98 单链可变片段的噬菌体展示文库而确定的,它保留了 CD98 的生理功能,并激发了广谱可结晶片段(Fc)介导的抗肿瘤活性(需要免疫球蛋白的 Fcγ 受体、巨噬细胞、树突状细胞和 CD8+ T 细胞以及先天性免疫系统和适应性免疫系统的其他成分)。我们还发现,通过解决抗体-CD98 复合物的结构而产生的具有 pH 依赖性结合的抗 CD98 抗体变体显示出更强的肿瘤特异性活性和药代动力学。一种具有 pH 依赖性结合的抗 CD98 抗体在 CD98 人源化小鼠建立的多个异种移植和合成肿瘤模型中激发了肿瘤特异性 Fc 介导的抗肿瘤活性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Biomedical Engineering
Medicine-Medicine (miscellaneous)
CiteScore
45.30
自引率
1.10%
发文量
138
期刊介绍:
Nature Biomedical Engineering is an online-only monthly journal that was launched in January 2017. It aims to publish original research, reviews, and commentary focusing on applied biomedicine and health technology. The journal targets a diverse audience, including life scientists who are involved in developing experimental or computational systems and methods to enhance our understanding of human physiology. It also covers biomedical researchers and engineers who are engaged in designing or optimizing therapies, assays, devices, or procedures for diagnosing or treating diseases. Additionally, clinicians, who make use of research outputs to evaluate patient health or administer therapy in various clinical settings and healthcare contexts, are also part of the target audience.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


