Advances in the structure, mechanism and targeting of chemoresistance-linked ABC transporters
IF 72.5
1区 医学
Q1 ONCOLOGY
引用次数: 0
Abstract
Cancer cells frequently display intrinsic or acquired resistance to chemically diverse anticancer drugs, limiting therapeutic success. Among the main mechanisms of this multidrug resistance is the overexpression of ATP-binding cassette (ABC) transporters that mediate drug efflux, and, specifically, ABCB1, ABCG2 and ABCC1 are known to cause cancer chemoresistance. High-resolution structures, biophysical and in silico studies have led to tremendous progress in understanding the mechanism of drug transport by these ABC transporters, and several promising therapies, including irradiation-based immune and thermal therapies, and nanomedicine have been used to overcome ABC transporter-mediated cancer chemoresistance. In this Review, we highlight the progress achieved in the past 5 years on the three transporters, ABCB1, ABCG2 and ABCC1, that are known to be of clinical importance. We address the molecular basis of their broad substrate specificity gleaned from structural information and discuss novel approaches to block the function of ABC transporters. Furthermore, genetic modification of ABC transporters by CRISPR–Cas9 and approaches to re-engineer amino acid sequences to change the direction of transport from efflux to import are briefly discussed. We suggest that current information regarding the structure, mechanism and regulation of ABC transporters should be used in clinical trials to improve the efficiency of chemotherapeutics for patients with cancer. This Review summarizes how the structural details that were revealed by cryo-electron microscopy and X-ray crystallography and insights into molecular basis of polyspecificity and mechanistic studies shaped the understanding of the role of ATP-binding cassette transporter in cancer multidrug resistance, culminating in new therapeutic approaches to sensitize multidrug-resistant cancer cells to conventional and targeted therapies.
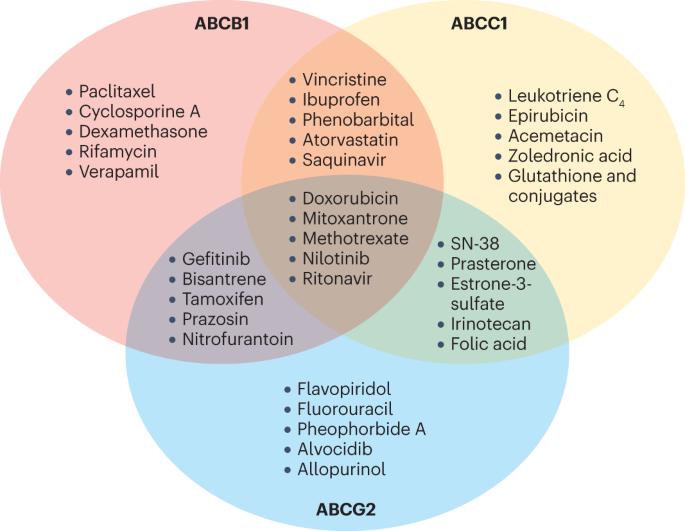
化学抗性ABC转运蛋白的结构、机制和靶向研究进展。
癌症细胞经常表现出对多种化学抗癌药物的内在或后天耐药性,限制了治疗的成功。这种多药耐药性的主要机制之一是ATP-结合盒(ABC)转运蛋白的过度表达,其介导药物流出,特别是ABCB1、ABCG2和ABCC1已知会导致癌症化疗耐药性。高分辨率结构、生物物理和计算机研究在理解这些ABC转运蛋白的药物转运机制方面取得了巨大进展,一些有前景的疗法,包括基于辐射的免疫和热疗法,以及纳米药物,已被用于克服ABC转运蛋白介导的癌症化疗耐药性。在这篇综述中,我们强调了过去5年在ABCB1、ABCG2和ABCC1这三种已知具有临床重要性的转运蛋白方面取得的进展。我们讨论了从结构信息中收集到的其广泛底物特异性的分子基础,并讨论了阻断ABC转运蛋白功能的新方法。此外,还简要讨论了CRISPR-Cas9对ABC转运蛋白的基因修饰,以及重新设计氨基酸序列以改变从流出到输入的转运方向的方法。我们建议,有关ABC转运蛋白的结构、机制和调节的最新信息应用于临床试验,以提高癌症患者的化疗效率。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Reviews Cancer
医学-肿瘤学
CiteScore
111.90
自引率
0.40%
发文量
97
审稿时长
6-12 weeks
期刊介绍:
Nature Reviews Cancer, a part of the Nature Reviews portfolio of journals, aims to be the premier source of reviews and commentaries for the scientific communities it serves. The correct abbreviation for abstracting and indexing purposes is Nat. Rev. Cancer. The international standard serial numbers (ISSN) for Nature Reviews Cancer are 1474-175X (print) and 1474-1768 (online). Unlike other journals, Nature Reviews Cancer does not have an external editorial board. Instead, all editorial decisions are made by a team of full-time professional editors who are PhD-level scientists. The journal publishes Research Highlights, Comments, Reviews, and Perspectives relevant to cancer researchers, ensuring that the articles reach the widest possible audience due to their broad scope.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


