A Randomized Double-blind Study to Assess the Skin Irritation and Sensitization Potential of a Once-weekly Donepezil Transdermal Delivery System in Healthy Volunteers.
IF 1.4
4区 医学
Q3 CLINICAL NEUROLOGY
Alzheimer Disease & Associated Disorders
Pub Date : 2023-10-01
Epub Date: 2023-09-11
DOI:10.1097/WAD.0000000000000578
引用次数: 0
Abstract
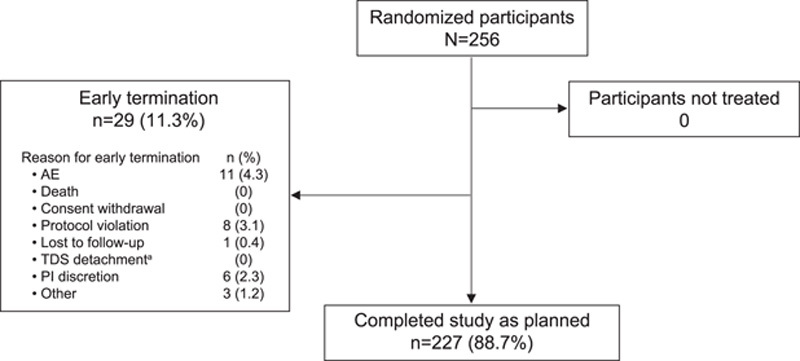
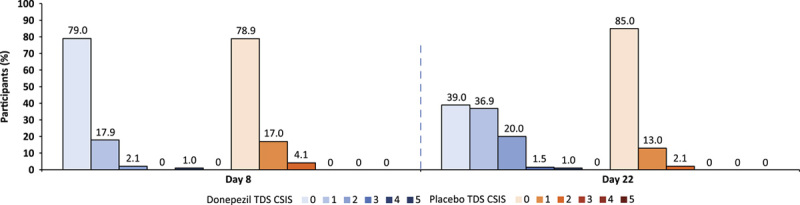
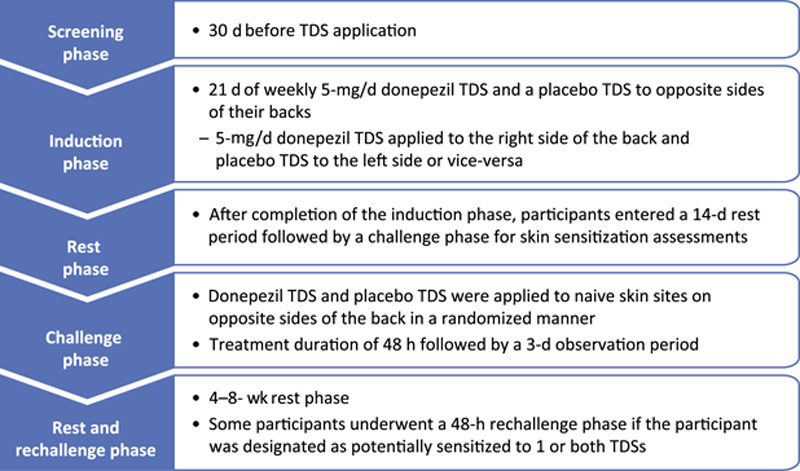
Background: A once-weekly donepezil transdermal delivery system (TDS; Adlarity; Corium, LLC) is indicated for the treatment of mild, moderate, and severe dementia of the Alzheimer type. Methods: In this placebo-controlled, randomized, double-blind phase 1 trial, healthy volunteers aged 40 years or older were randomized to receive a placebo and donepezil TDS and were evaluated for the primary endpoints of skin irritation and sensitization potential. Skin irritation was scored. Results: Two hundred fifty-six participants were randomized and received ≥1 dose of any treatment. After the first weekly TDS application, no skin irritation or minimal irritation was evident between donepezil and placebo TDSs. At the third weekly TDS application, for donepezil TDS, the average of the mean combined skin irritation score was 0.55 of a possible maximum of 7, indicating none to minimal skin irritation, and for placebo, the score was 0.19, indicating no skin irritation. Of 198 participants, 4 (2.0%) were considered potentially sensitized to donepezil TDS, and 0 were potentially sensitized to placebo TDS. Conclusion: Once-weekly 5-mg/d donepezil TDS demonstrated minimal skin irritation under conditions of use of 3 consecutive weekly patch applications to the same skin site and minimal sensitization potential.
一项随机双盲研究评估健康志愿者每周一次的多奈哌齐透皮给药系统的皮肤刺激和致敏潜力。
研究背景:每周一次的多奈哌齐透皮给药系统(TDS;Adlarity;Corium, LLC)适用于治疗轻度、中度和重度阿尔茨海默型痴呆。方法:在这项安慰剂对照、随机、双盲的1期试验中,年龄在40岁或以上的健康志愿者随机接受安慰剂和多奈哌齐TDS,并评估皮肤刺激和致敏潜力的主要终点。对皮肤刺激进行评分。结果:256名参与者随机接受≥1剂量的任何治疗。在第一周TDS应用后,多奈哌齐和安慰剂TDS之间没有明显的皮肤刺激或最小刺激。在第三周TDS应用时,对于多奈哌齐TDS,平均联合皮肤刺激评分的平均值为0.55(可能的最大值为7),表明没有到最小的皮肤刺激,对于安慰剂,得分为0.19,表明没有皮肤刺激。198名参与者中,4人(2.0%)被认为对多奈哌齐TDS潜在致敏,0人对安慰剂TDS潜在致敏。结论:每周一次5mg /d多奈哌齐TDS在使用连续3周的贴片应用于同一皮肤部位的情况下表现出最小的皮肤刺激和最小的致敏潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
3.10
自引率
4.80%
发文量
88
期刊介绍:
Alzheimer Disease & Associated Disorders is a peer-reviewed, multidisciplinary journal directed to an audience of clinicians and researchers, with primary emphasis on Alzheimer disease and associated disorders. The journal publishes original articles emphasizing research in humans including epidemiologic studies, clinical trials and experimental studies, studies of diagnosis and biomarkers, as well as research on the health of persons with dementia and their caregivers. The scientific portion of the journal is augmented by reviews of the current literature, concepts, conjectures, and hypotheses in dementia, brief reports, and letters to the editor.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


