Dupilumab-Induced Lichen Planus: A Case with Oral and Cutaneous Eruptions.
IF 0.9
Q4 DERMATOLOGY
引用次数: 2
Abstract
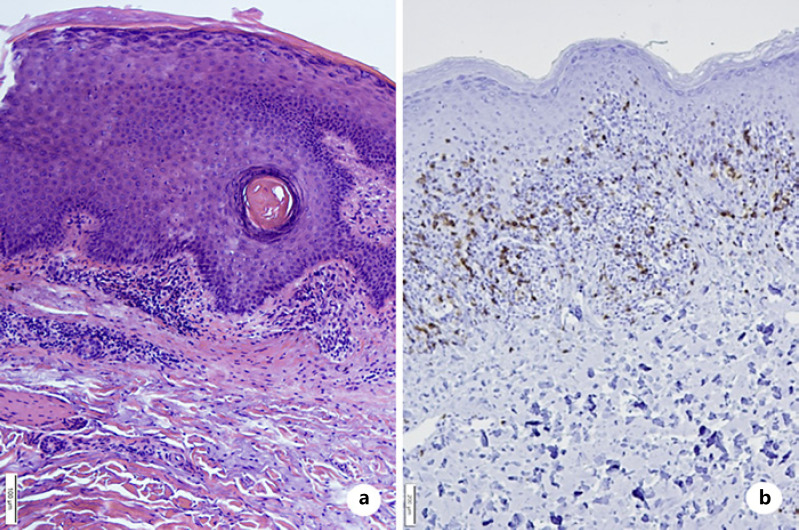
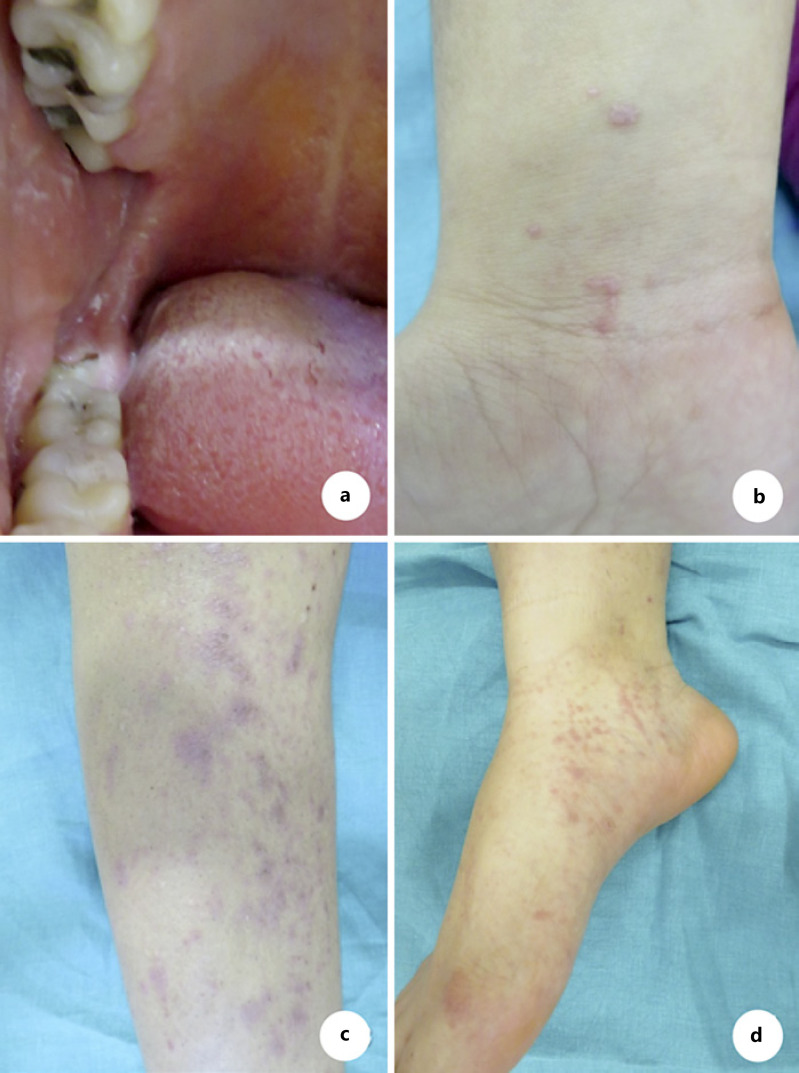
Lichen planus is a chronic, inflammatory, immune-mediated dermatosis affecting the patient’s skin, scalp, mucous membranes, and nails. Drug-induced lichen planus is described after the administration of antimalarials, ß-blockers, methyldopa, NSAIDs, penicillamines, and sodium aurothiomalate. The use of biologicals such as adalimumab, etanercept, and infliximab has also been linked with the appearance of lichenoid eruptions in the recent past. In this case, we report on a patient developing oral and cutaneous lichen planus after the administration of dupilumab. The lichenoid lesions occurred after 11 months of the drug’s administration and involved the buccal walls, trunk, and extremities. Dupilumab had been administered in an effort to counter severe atopic dermatitis exacerbations. Dupilumab is associated with a downregulation of T-helper 2 cell activation by blocking the Interleukin-4/Interleukin-13 pathway, so leading to a TH1/TH2 imbalance. This imbalance may cause a shift toward a TH1-mediated immune response and be an explanation for the drug-induced lichen planus. Dupilumab was discontinued, and the patient was treated with oral corticosteroids and UVB phototherapy, leading to a significant improvement in the lichen planus lesions.
杜匹单抗诱导的扁平苔藓:1例口腔和皮肤皮疹。
扁平苔藓是一种慢性、炎症性、免疫介导的皮肤病,影响患者的皮肤、头皮、粘膜和指甲。药物性扁平苔藓是在服用抗疟药、ß-阻滞剂、甲基多巴、非甾体抗炎药、青霉胺和金硫硫酸钠后发生的。阿达木单抗、依那西普和英夫利昔单抗等生物制剂的使用也与最近出现的地衣样疹有关。在这种情况下,我们报告的患者发展口腔和皮肤扁平苔藓后给予杜匹单抗。地衣样病变发生在给药11个月后,累及颊壁、鼻干和四肢。Dupilumab已被用于对抗严重的特应性皮炎恶化。Dupilumab通过阻断白细胞介素-4/白细胞介素-13通路,与t -辅助性2细胞活化下调相关,从而导致TH1/TH2失衡。这种不平衡可能导致向th1介导的免疫反应转变,并可以解释药物诱导的扁平苔藓。停用杜匹单抗,患者接受口服皮质类固醇和UVB光疗治疗,导致扁平苔藓病变显著改善。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


