The inhibition of MDM2 slows cell proliferation and activates apoptosis in ADPKD cell lines
Abstract
Introduction
Autosomal dominant polycystic kidney disease (ADPKD) is characterised by progressive cysts formation and renal enlargement that in most of cases leads to end stage of renal disease (ESRD). This pathology is caused by mutations of either PKD1 or PKD2 genes that encode for polycystin-1 (PC1) and polycystin-2 (PC2), respectively. These proteins function as receptor-channel complex able to regulate calcium homeostasis. PKD1/2 loss of function impairs different signalling pathways including cAMP and mTOR that are considered therapeutic targets for this disease. In fact, Tolvaptan, a vasopressin-2 antagonist that reduces cAMP levels, is the only drug approved for ADPKD treatment. Nevertheless, some ADPKD patients developed side effects in response to Tolvaptan including liver damage. Conversely, mTOR inhibitors that induced disease regression in ADPKD animal models failed the clinical trials.
Results
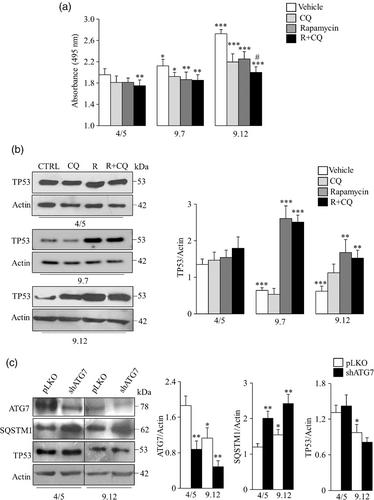
Here, we show that the inhibition of mTOR causes the activation of autophagy in ADPKD cells that could reduce therapy effectiveness by drug degradation through the autophagic vesicles. Consistently, the combined treatment with rapamycin and chloroquine, an autophagy inhibitor, potentiates the decrease of cell proliferation induced by rapamycin. To overcome the dangerous activation of autophagy by mTOR inhibition, we targeted MDM2 (a downstream effector of mTOR signalling) that is involved in TP53 degradation by using RG7112, a small-molecule MDM2 inhibitor used for the treatment of haematologic malignancies. The inhibition of MDM2 by RG7112 prevents TP53 degradation and increases p21 expression leading to the decrease of cell proliferation and the activation of apoptosis.
Conclusion
The targeting of MDM2 by RG7112 might represent a new therapeutic option for the treatment of ADPKD.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


