Evaluation of platelet additive solution for prolonging storage of functional canine platelet concentrate
Abstract
Objective
To assess storage lesion development, platelet function, and bacterial growth in canine platelet concentrates (PCs) stored in a platelet additive solution (PAS) or a plasma control at 4°C for 21 days.
Design
Prospective, ex vivo, experimental controlled study.
Setting
University veterinary teaching hospital.
Animals
Ten units of canine PCs collected from blood bank donations.
Interventions
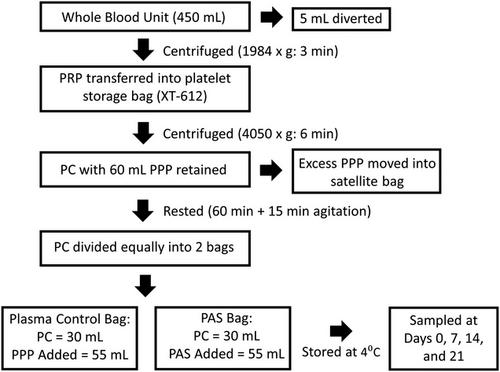
The PCs were separated into 2 bags, 1 containing 100% plasma and the other containing 35% plasma and 65% of a PAS (Plasma-Lyte A), and stored at 4°C for 21 days. At days 0, 7, 14, and 21, PCs were analyzed for the presence of swirling, aggregate formation, platelet counts, platelet indices, glucose, lactate, lactate dehydrogenase, Pvco2, Pvo2, aggregation via light aggregometry, activation percentages using flow cytometry, and bacterial growth.
Measurements and main results
Cold-stored PCs in both PAS and plasma control maintained mean pH >6.8 and mean lactate <9.0 mmol/L over 21 days, with no difference in glucose utilization. Swirl was maintained in both solutions for most days (76/80 combined total samples), with no difference in aggregate formation between solutions. The Pvco2 was higher in plasma on all days (P < 0.001), with no difference in Pvo2. Platelet indices did not reflect significant storage lesion development in either solution. Lactate dehydrogenase did not differ between solutions but did increase from day 7 to day 21. Mean maximal aggregation percentage was reduced overall but with no significant difference between solutions. The only observed difference in mean activation percentage between solutions was in PAS on day 7, which was significantly higher than plasma (P < 0.05). No bacterial growth occurred during storage.
Conclusions
Cold storage in PAS and plasma allowed PCs to be stored for up to 21 days with minimal storage lesion development, maintenance of platelet function, limited platelet activation, and no bacterial growth within stored bags.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


