Remodeling oncogenic transcriptomes by small molecules targeting NONO
IF 13.7
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 2
Abstract
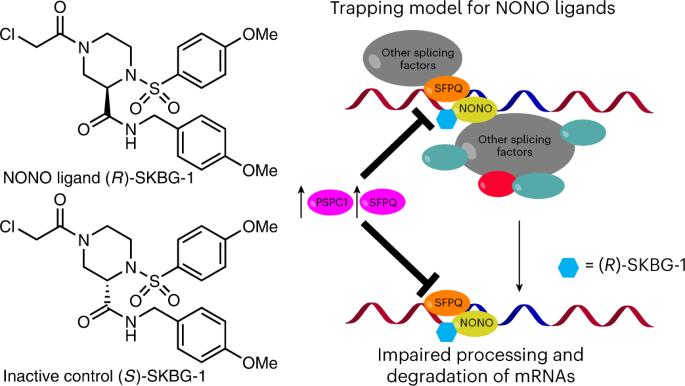
Much of the human proteome is involved in mRNA homeostasis, but most RNA-binding proteins lack chemical probes. Here we identify electrophilic small molecules that rapidly and stereoselectively decrease the expression of transcripts encoding the androgen receptor and its splice variants in prostate cancer cells. We show by chemical proteomics that the compounds engage C145 of the RNA-binding protein NONO. Broader profiling revealed that covalent NONO ligands suppress an array of cancer-relevant genes and impair cancer cell proliferation. Surprisingly, these effects were not observed in cells genetically disrupted for NONO, which were instead resistant to NONO ligands. Reintroduction of wild-type NONO, but not a C145S mutant, restored ligand sensitivity in NONO-disrupted cells. The ligands promoted NONO accumulation in nuclear foci and stabilized NONO–RNA interactions, supporting a trapping mechanism that may prevent compensatory action of paralog proteins PSPC1 and SFPQ. These findings show that NONO can be co-opted by covalent small molecules to suppress protumorigenic transcriptional networks. Integrated phenotypic screening and activity-based protein profiling identifies small molecules that decrease the expression of oncogenic transcription factors and suppress cancer cell growth by covalently targeting the RNA-binding protein NONO.
通过靶向 NONO 的小分子重塑致癌转录组
人类蛋白质组的大部分都参与了 mRNA 的平衡,但大多数 RNA 结合蛋白都缺乏化学探针。在这里,我们发现了亲电小分子,它们能快速、立体选择性地降低前列腺癌细胞中编码雄激素受体及其剪接变体的转录本的表达。我们通过化学蛋白质组学研究发现,这些化合物能与 RNA 结合蛋白 NONO 的 C145 结合。更广泛的分析表明,共价 NONO 配体抑制了一系列癌症相关基因,并损害了癌细胞的增殖。令人惊讶的是,在NONO基因被破坏的细胞中没有观察到这些效应,相反,这些细胞对NONO配体具有抗性。重新引入野生型 NONO 而非 C145S 突变体可恢复 NONO 基因中断细胞对配体的敏感性。配体促进了 NONO 在核病灶中的积累,并稳定了 NONO-RNA 的相互作用,支持了一种可能阻止旁系蛋白 PSPC1 和 SFPQ 补偿作用的捕获机制。这些研究结果表明,共价小分子可以利用 NONO 来抑制原癌基因转录网络。综合表型筛选和基于活性的蛋白质图谱分析,确定了通过共价靶向 RNA 结合蛋白 NONO 来降低致癌转录因子表达和抑制癌细胞生长的小分子。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature chemical biology
生物-生化与分子生物学
CiteScore
23.90
自引率
1.40%
发文量
238
审稿时长
12 months
期刊介绍:
Nature Chemical Biology stands as an esteemed international monthly journal, offering a prominent platform for the chemical biology community to showcase top-tier original research and commentary. Operating at the crossroads of chemistry, biology, and related disciplines, chemical biology utilizes scientific ideas and approaches to comprehend and manipulate biological systems with molecular precision.
The journal embraces contributions from the growing community of chemical biologists, encompassing insights from chemists applying principles and tools to biological inquiries and biologists striving to comprehend and control molecular-level biological processes. We prioritize studies unveiling significant conceptual or practical advancements in areas where chemistry and biology intersect, emphasizing basic research, especially those reporting novel chemical or biological tools and offering profound molecular-level insights into underlying biological mechanisms.
Nature Chemical Biology also welcomes manuscripts describing applied molecular studies at the chemistry-biology interface due to the broad utility of chemical biology approaches in manipulating or engineering biological systems. Irrespective of scientific focus, we actively seek submissions that creatively blend chemistry and biology, particularly those providing substantial conceptual or methodological breakthroughs with the potential to open innovative research avenues. The journal maintains a robust and impartial review process, emphasizing thorough chemical and biological characterization.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


