Light-Mediated Electrochemical Synthesis of Manganese Oxide Enhances Its Stability for Water Oxidation
IF 4.8
Q2 NANOSCIENCE & NANOTECHNOLOGY
引用次数: 0
Abstract
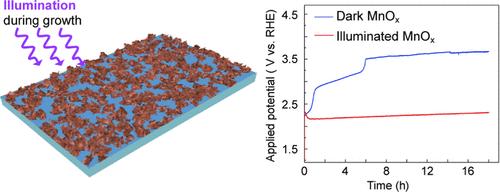
New methods are needed to increase the activity and stability of earth-abundant catalysts for electrochemical water splitting to produce hydrogen fuel. Electrodeposition has been previously used to synthesize manganese oxide films with a high degree of disorder and a mixture of oxidation states for Mn, which has led to electrocatalysts with high activity but low stability for the oxygen evolution reaction (OER) at high current densities. In this study, we show that multipotential electrodeposition of manganese oxide under illumination produces nanostructured films with significantly higher stability for the OER compared to films grown under otherwise identical conditions in the dark. Manganese oxide films grown by multipotential deposition under illumination sustain a current density of 10 mA/cm2 at 2.2 V versus reversible hydrogen electrode for 18 h (pH 13). Illumination does not enhance the activity or stability of manganese oxide films grown using a constant potential, and films grown by multipotential deposition in the dark undergo a complete loss of activity within 1 h of electrolysis. Electrochemical and structural characterization indicate that photoexcitation of the films during growth reduces Mn ions and changes the content and structure of intercalated potassium ions and water molecules in between the disordered layers of birnessite-like sheets of MnOx, which stabilizes the nanostructured film during electrocatalysis. These results demonstrate that combining multiple external stimuli (i.e., light and an external potential) can induce structural changes not attainable by either stimulus alone to make earth-abundant catalysts more active and stable for important chemical transformations such as water oxidation.
光介导的电化学合成提高氧化锰的水氧化稳定性
需要新的方法来提高富含地球的催化剂的活性和稳定性,用于电化学水分解以生产氢燃料。电沉积先前已用于合成具有高度无序性和Mn氧化态混合物的氧化锰膜,这导致电催化剂在高电流密度下对析氧反应(OER)具有高活性但低稳定性。在这项研究中,我们表明,与在黑暗中相同条件下生长的膜相比,在照明下氧化锰的多电位电沉积产生的纳米结构膜对OER具有显著更高的稳定性。在照明下通过多电位沉积生长的氧化锰膜在2.2V下相对于可逆氢电极维持10mA/cm2的电流密度18小时(pH 13)。光照不会增强使用恒定电势生长的氧化锰膜的活性或稳定性,并且通过在黑暗中的多电势沉积生长的膜在电解1小时内经历活性的完全丧失。电化学和结构表征表明,在生长过程中,薄膜的光激发减少了Mn离子,并改变了MnOx类水镁石片无序层之间嵌入的钾离子和水分子的含量和结构,这在电催化过程中稳定了纳米结构薄膜。这些结果表明,将多种外部刺激(即光和外部电势)相结合,可以引起单独任何一种刺激都无法实现的结构变化,从而使富含地球的催化剂对重要的化学转化(如水氧化)更具活性和稳定性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Nanoscience Au
材料科学、纳米科学-
CiteScore
4.20
自引率
0.00%
发文量
0
期刊介绍:
ACS Nanoscience Au is an open access journal that publishes original fundamental and applied research on nanoscience and nanotechnology research at the interfaces of chemistry biology medicine materials science physics and engineering.The journal publishes short letters comprehensive articles reviews and perspectives on all aspects of nanoscience and nanotechnology:synthesis assembly characterization theory modeling and simulation of nanostructures nanomaterials and nanoscale devicesdesign fabrication and applications of organic inorganic polymer hybrid and biological nanostructuresexperimental and theoretical studies of nanoscale chemical physical and biological phenomenamethods and tools for nanoscience and nanotechnologyself- and directed-assemblyzero- one- and two-dimensional materialsnanostructures and nano-engineered devices with advanced performancenanobiotechnologynanomedicine and nanotoxicologyACS Nanoscience Au also publishes original experimental and theoretical research of an applied nature that integrates knowledge in the areas of materials engineering physics bioscience and chemistry into important applications of nanomaterials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


