The phosphatidylinositol (4,5)-bisphosphate-Rab35 axis regulates migrasome formation
IF 25.9
1区 生物学
Q1 CELL BIOLOGY
引用次数: 3
Abstract
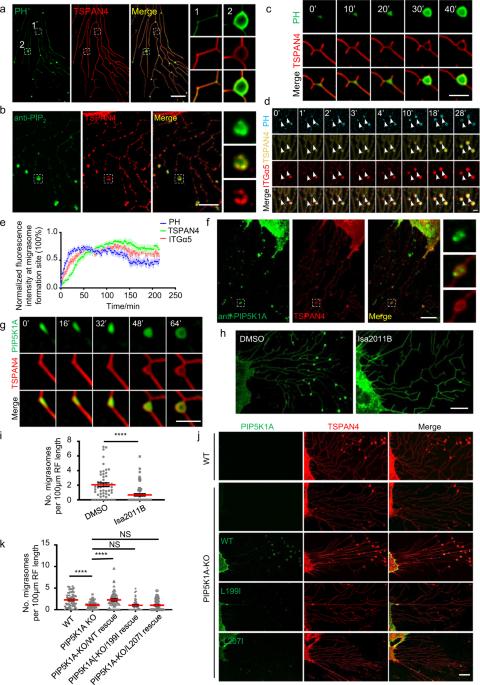
Migrasomes are recently discovered organelles, which are formed on the ends or branch points of retraction fibers at the trailing edge of migrating cells. Previously, we showed that recruitment of integrins to the site of migrasome formation is essential for migrasome biogenesis. In this study, we found that prior to migrasome formation, PIP5K1A, a PI4P kinase which converts PI4P into PI(4,5)P2, is recruited to migrasome formation sites. The recruitment of PIP5K1A results in generation of PI(4,5)P2 at the migrasome formation site. Once accumulated, PI(4,5)P2 recruits Rab35 to the migrasome formation site by interacting with the C-terminal polybasic cluster of Rab35. We further demonstrated that active Rab35 promotes migrasome formation by recruiting and concentrating integrin α5 at migrasome formation sites, which is likely mediated by the interaction between integrin α5 and Rab35. Our study identifies the upstream signaling events orchestrating migrasome biogenesis.
磷脂酰肌醇(4,5)-二磷酸-Rab35 轴调控迁移体的形成
迁移体是最近发现的细胞器,形成于迁移细胞后缘的回缩纤维末端或分支点。此前,我们发现整合素在移行体形成部位的招募对移行体的生物生成至关重要。在本研究中,我们发现在移行体形成之前,PIP5K1A(一种将PI4P转化为PI(4,5)P2的PI4P激酶)被招募到移行体形成位点。PIP5K1A 的招募会在迁移体形成部位产生 PI(4,5)P2。一旦积累,PI(4,5)P2 就会通过与 Rab35 的 C 端多聚基团相互作用,将 Rab35 募集到迁移体形成位点。我们进一步证实,活跃的Rab35通过招募整合素α5并将其集中在迁移体形成位点,从而促进迁移体的形成,这可能是由整合素α5和Rab35之间的相互作用介导的。我们的研究确定了协调迁移体生物形成的上游信号事件。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell Research
生物-细胞生物学
CiteScore
53.90
自引率
0.70%
发文量
2420
审稿时长
2.3 months
期刊介绍:
Cell Research (CR) is an international journal published by Springer Nature in partnership with the Center for Excellence in Molecular Cell Science, Chinese Academy of Sciences (CAS). It focuses on publishing original research articles and reviews in various areas of life sciences, particularly those related to molecular and cell biology. The journal covers a broad range of topics including cell growth, differentiation, and apoptosis; signal transduction; stem cell biology and development; chromatin, epigenetics, and transcription; RNA biology; structural and molecular biology; cancer biology and metabolism; immunity and molecular pathogenesis; molecular and cellular neuroscience; plant molecular and cell biology; and omics, system biology, and synthetic biology. CR is recognized as China's best international journal in life sciences and is part of Springer Nature's prestigious family of Molecular Cell Biology journals.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


