Age-associated disparity in phagocytic clearance affects the efficacy of cancer nanotherapeutics
IF 38.1
1区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
Abstract
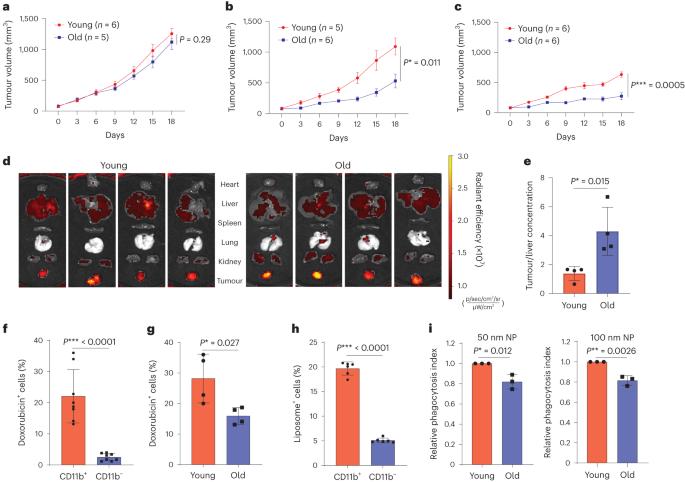
Nanomedicines have been approved to treat multiple human diseases. However, clinical adoption of nanoformulated agents is often hindered by concerns about hepatic uptake and clearance, a process that is not fully understood. Here we show that the antitumour efficacy of cancer nanomedicine exhibits an age-associated disparity. Tumour delivery and treatment outcomes are superior in old versus young mice, probably due to an age-related decline in the ability of hepatic phagocytes to take up and remove nanoparticles. Transcriptomic- and protein-level analysis at the single-cell and bulk levels reveals an age-associated decrease in the numbers of hepatic macrophages that express the scavenger receptor MARCO in mice, non-human primates and humans. Therapeutic blockade of MARCO is shown to decrease the phagocytic uptake of nanoparticles and improve the antitumour effect of clinically approved cancer nanotherapeutics in young but not aged mice. Together, these results reveal an age-associated disparity in the phagocytic clearance of nanotherapeutics that affects their antitumour response, thus providing a strong rationale for an age-appropriate approach to cancer nanomedicine. Here, the authors find a decrease in hepatic phagocytic uptake of nanoparticles in old mice due to age-associated downregulation of the scavenger receptor MARCO, which led to improved tumour delivery and antitumour efficacy of cancer nanomedicine, showing the need to consider age as a factor in therapeutics.
与Ag相关的吞噬清除差异影响癌症纳米疗法的疗效。
纳米药物已被批准用于治疗多种人类疾病。然而,由于对肝脏摄取和清除的担忧,纳米制剂的临床应用往往受到阻碍,这一过程尚不完全清楚。在这里,我们表明癌症纳米药物的抗肿瘤疗效表现出与年龄相关的差异。与年轻小鼠相比,老年小鼠的肿瘤递送和治疗效果更好,这可能是由于肝吞噬细胞吸收和清除纳米颗粒的能力与年龄相关而下降。单细胞和大体积水平的转录组学和蛋白质水平分析显示,在小鼠、非人灵长类动物和人类中,表达清道夫受体MARCO的肝巨噬细胞数量与年龄相关地减少。MARCO的治疗阻断显示可降低纳米颗粒的吞噬摄取,并改善临床批准的癌症纳米疗法在年轻但未衰老小鼠中的抗肿瘤作用。总之,这些结果揭示了影响其抗肿瘤反应的纳米治疗剂吞噬清除率的年龄相关差异,从而为癌症纳米药物的年龄适当方法提供了有力的依据。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature nanotechnology
工程技术-材料科学:综合
CiteScore
59.70
自引率
0.80%
发文量
196
审稿时长
4-8 weeks
期刊介绍:
Nature Nanotechnology is a prestigious journal that publishes high-quality papers in various areas of nanoscience and nanotechnology. The journal focuses on the design, characterization, and production of structures, devices, and systems that manipulate and control materials at atomic, molecular, and macromolecular scales. It encompasses both bottom-up and top-down approaches, as well as their combinations.
Furthermore, Nature Nanotechnology fosters the exchange of ideas among researchers from diverse disciplines such as chemistry, physics, material science, biomedical research, engineering, and more. It promotes collaboration at the forefront of this multidisciplinary field. The journal covers a wide range of topics, from fundamental research in physics, chemistry, and biology, including computational work and simulations, to the development of innovative devices and technologies for various industrial sectors such as information technology, medicine, manufacturing, high-performance materials, energy, and environmental technologies. It includes coverage of organic, inorganic, and hybrid materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


