The PTPIP51 TPR-Domain: A Novel Lipid Transfer Domain?
Contact (Thousand Oaks (Ventura County, Calif.))
Pub Date : 2021-01-01
DOI:10.1177/25152564211056192
引用次数: 2
Abstract
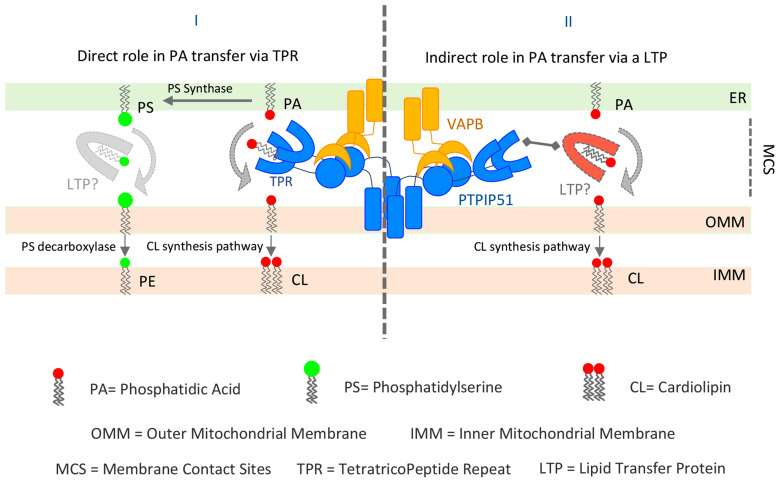
During the last decade, mitochondria-associated ER membranes (MAMs) have emerged as critical signaling, metabolic and trafficking hubs involved in the regulation of multiple cellular processes including autophagy, inflammation, signaling and apoptosis (Csordas et al., 2018; Phillips & Voeltz, 2016; Rowland & Voeltz, 2012). MAMs are zones of close membrane proximity where the ER and mitochondria membranes are tethered by multiple linker proteins, allowing direct exchange of key metabolites and ions, such as lipids and Ca, between these two organelles that are not connected by the classical vesicle-transport routes. While the mechanism and proteins involved in Ca transport at the MAMs are well characterized, our knowledge on how lipids are exchanged between these two organelles is still rudimentary, especially in metazoa, as the lipid transfer proteins (LTPs) have just started to be identified. Writing in EMBO Reports, Yeo and colleagues (Yeo et al., 2021) reveal a new role of the mitochondrial protein PTPIP51 (also known as Regulator of Microtubule Dynamics, RMD3) in lipid transfer at MAMs. PTPIP51 has been previously shown to be a tether that bridges ER and mitochondria membranes via interaction with the ER protein VAPB, thus facilitating Ca transport to mitochondria (Stoica et al., 2014). However, the exact biochemical function of PTPIP51 beyond ER-mitochondria tethering had so far been unclear. PTPIP51 possesses tandem FFAT motifs involved in the binding to VAP (Di Mattia et al., 2020; Mikitova & Levine, 2012), a coiled coil (CC) domain and a large C-terminal globular domain, the tetratricopeptide repeat (TPR) whose role in PTPIP51 function remains enigmatic. With over 100 TPR structures deposited in the protein data bank, the TPR domain has established itself as a major protein-protein interaction module (Blatch & Lassle, 1999). A diverse array of protein ligands has been seen to bind within the TPR cleft. These do not share any common sequence or secondary structure. Moreover, the diversity of the ligand and the amino-acid residues that line the binding cleft of the TPR domain produce highly specific TPR binding domains. Yeo and colleagues reveal a novel role for the TPR of PTPIP51 in binding and transferring phospholipids that is unusual if compared to the established proteinprotein interaction mode of other TPR domains. On this line, Yeo and colleagues show that interaction of PTPIP51 with VAPB is not mediated by the TPR domain but by the tandem FFAT-like motif. Then, they provide evidence that suggests that the PTPIP51 TPR-domain is involved in phospholipid binding and transfer at MAMs. The authors present the X-ray structure of the TPR domain of PTPIP51 and biochemical evidence in vitro and in situ for a lipid binding and transfer function. They propose that the PTPIP51−VAPB complex might be the counterpart of the yeast ER–mitochondria encounter structure (ERMES) complex, responsible for phospholipid transportation at MAMs (AhYoung et al., 2015; Kornmann et al., 2009; Tatsuta et al., 2014). Although other LTPs are also present at contact sites between ER and mitochondria (i.e. ORP5/8, PDZD8, VPS13A), the unexpected finding that the PTPIP51 TPR domain could be involved in lipid trafficking at the same interface is very intriguing and naturally deserves closer scrutiny. However, before we discuss this, it would be useful to draw some information from structures that are known to be lipid trafficking proteins (LTPs) at regions of close membrane apposition, including MAMs. There are several major families of LTPs that localize at membrane contact sites, each containing a core lipid-binding/transfer domain (Giordano, 2018), that include oxysterol-binding protein (OSBP)-related proteins (ORD), START, START/VASt, PITPs, PRELI-like, and SMP domains, that collectively act as tethers, lipid sensors, or transporters at multiple contact sites (Giordano, 2018). Looking at examples of such
PTPIP51 tpr结构域:一个新的脂质转移结构域?
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


