A standard 96-well based high throughput microfluidic perfusion biofilm reactor for in situ optical analysis
Abstract
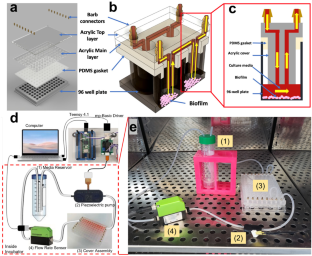
Biofilm infections represent a major public health threat due to their high tolerance to antimicrobials and the lack of specific anti-biofilm drugs. To develop such drugs, it is crucial to have high-throughput biofilm growth systems that can emulate in vivo conditions without the cost and complexity of animal models. However, no current biofilm reactor can provide in vivo-like conditions in a high throughput standard microtiter format. This paper demonstrates a novel high-throughput (HT) microfluidic perfusion biofilm reactor (HT-μPBR) compatible with a standard 96-well microtiter plate for in situ optical analysis. A snap-on liquid-tight cover for standard microtiter plates was designed and fabricated with fluidic channels to provide closed-loop recirculating perfusion. Our system takes steps toward providing in vivo-like conditions with controlled shear stress and nutrient delivery. We describe the system fabrication and usage in optical analysis of biomass and viability of Escherichia coli (E. coli) biofilms. The HT-μPBR was set to perfuse at 1 mL/min corresponding to an average shear rate of approximately \(5.7{\mathrm{s}}^{-1}\) on the bottom surface of a single well. Biofilms were detected on well plate bottoms and measured using a fluorescence microscope and plate reader to determine biomass and viability. Samples cultured in the HT-μPBR showed increased biomass while maintaining viability after 24 h. The HT-μPBR can further be combined with HT antibiotic susceptibility testing and additional optical techniques such as time-lapse imaging to improve understanding of the drug reaction mechanism as well as the optimization of drug combinations and delivery profiles.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


