Structural insight into selectivity of amylin and calcitonin receptor agonists
IF 12.9
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Amylin receptors (AMYRs), heterodimers of the calcitonin receptor (CTR) and one of three receptor activity-modifying proteins, are promising obesity targets. A hallmark of AMYR activation by Amy is the formation of a ‘bypass’ secondary structural motif (residues S19–P25). This study explored potential tuning of peptide selectivity through modification to residues 19–22, resulting in a selective AMYR agonist, San385, as well as nonselective dual amylin and calcitonin receptor agonists (DACRAs), with San45 being an exemplar. We determined the structure and dynamics of San385-bound AMY3R, and San45 bound to AMY3R or CTR. San45, via its conjugated lipid at position 21, was anchored at the edge of the receptor bundle, enabling a stable, alternative binding mode when bound to the CTR, in addition to the bypass mode of binding to AMY3R. Targeted lipid modification may provide a single intervention strategy for design of long-acting, nonselective, Amy-based DACRAs with potential anti-obesity effects. Cryo-EM structure and dynamics analysis provides a conformational mechanism for tuning of selectivity between calcitonin and amylin receptors through targeted lipid modification of residues 19–22 within the ‘bypass’ motif of amylin peptides.
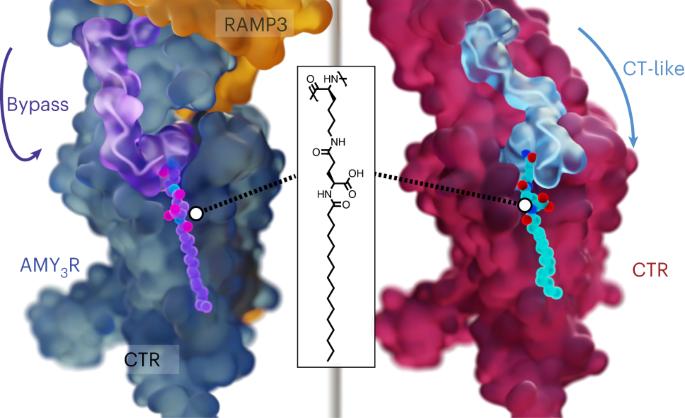
从结构上洞察淀粉样蛋白和降钙素受体激动剂的选择性。
淀粉受体(AMYRs)是降钙素受体(CTR)和三种受体活性修饰蛋白之一的异二聚体,是很有希望的肥胖症靶点。艾米激活 AMYR 的一个特征是形成一个 "旁路 "二级结构基团(残基 S19-P25)。本研究探索了通过修饰残基 19-22 来调整多肽选择性的可能性,从而产生了一种选择性 AMYR 激动剂 San385,以及一种非选择性淀粉和降钙素受体双重激动剂(DACRAs),其中 San45 就是一个范例。我们测定了与 San385 结合的 AMY3R 以及与 AMY3R 或 CTR 结合的 San45 的结构和动力学。San45 通过其位于 21 位的共轭脂质锚定在受体束边缘,除了与 AMY3R 的旁路结合模式外,还能在与 CTR 结合时实现稳定的替代结合模式。靶向脂质修饰可能为设计具有潜在抗肥胖作用的长效、非选择性、基于艾米的 DACRA 提供了一种单一的干预策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature chemical biology
生物-生化与分子生物学
CiteScore
23.90
自引率
1.40%
发文量
238
审稿时长
12 months
期刊介绍:
Nature Chemical Biology stands as an esteemed international monthly journal, offering a prominent platform for the chemical biology community to showcase top-tier original research and commentary. Operating at the crossroads of chemistry, biology, and related disciplines, chemical biology utilizes scientific ideas and approaches to comprehend and manipulate biological systems with molecular precision.
The journal embraces contributions from the growing community of chemical biologists, encompassing insights from chemists applying principles and tools to biological inquiries and biologists striving to comprehend and control molecular-level biological processes. We prioritize studies unveiling significant conceptual or practical advancements in areas where chemistry and biology intersect, emphasizing basic research, especially those reporting novel chemical or biological tools and offering profound molecular-level insights into underlying biological mechanisms.
Nature Chemical Biology also welcomes manuscripts describing applied molecular studies at the chemistry-biology interface due to the broad utility of chemical biology approaches in manipulating or engineering biological systems. Irrespective of scientific focus, we actively seek submissions that creatively blend chemistry and biology, particularly those providing substantial conceptual or methodological breakthroughs with the potential to open innovative research avenues. The journal maintains a robust and impartial review process, emphasizing thorough chemical and biological characterization.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


