Cooperative phagocytosis of solid tumours by macrophages triggers durable anti-tumour responses
IF 26.8
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 5
Abstract
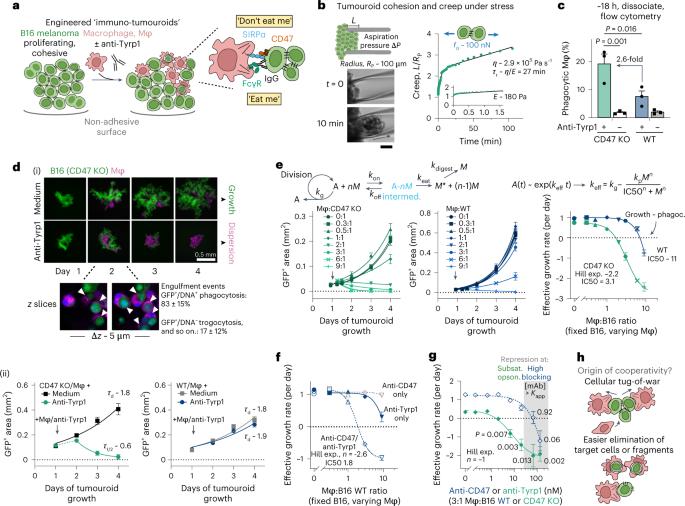
In solid tumours, the abundance of macrophages is typically associated with a poor prognosis. However, macrophage clusters in tumour-cell nests have been associated with survival in some tumour types. Here, by using tumour organoids comprising macrophages and cancer cells opsonized via a monoclonal antibody, we show that highly ordered clusters of macrophages cooperatively phagocytose cancer cells to suppress tumour growth. In mice with poorly immunogenic tumours, the systemic delivery of macrophages with signal-regulatory protein alpha (SIRPα) genetically knocked out or else with blockade of the CD47–SIRPα macrophage checkpoint was combined with the monoclonal antibody and subsequently triggered the production of endogenous tumour-opsonizing immunoglobulin G, substantially increased the survival of the animals and helped confer durable protection from tumour re-challenge and metastasis. Maximizing phagocytic potency by increasing macrophage numbers, by tumour-cell opsonization and by disrupting the phagocytic checkpoint CD47–SIRPα may lead to durable anti-tumour responses in solid cancers. Durable anti-tumour responses can be triggered by maximizing the cooperative phagocytic potency of macrophages through the disruption of the CD47–SIRPα macrophage checkpoint and by delivering a tumour-opsonizing monoclonal antibody.
巨噬细胞对实体瘤的协同吞噬作用可引发持久的抗肿瘤反应。
在实体瘤中,巨噬细胞的丰度通常与预后不良有关。然而,肿瘤细胞巢中的巨噬细胞簇与某些肿瘤类型的生存有关。在这里,通过使用包括巨噬细胞和通过单克隆抗体调理的癌症细胞的肿瘤类器官,我们表明高度有序的巨噬细胞簇协同吞噬癌症细胞以抑制肿瘤生长。在免疫原性较差的肿瘤小鼠中,通过基因敲除或阻断CD47-SIRPα巨噬细胞检查点的信号调节蛋白α(SIRPα)的巨噬细胞的全身递送与单克隆抗体结合,随后触发内源性肿瘤调理免疫球蛋白G的产生,大大提高了动物的存活率,并有助于提供持久的保护,防止肿瘤再次侵袭和转移。通过增加巨噬细胞数量、肿瘤细胞调理和破坏吞噬检查点CD47-SIRPα来最大限度地提高吞噬能力,可能会在实体癌中产生持久的抗肿瘤反应。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Biomedical Engineering
Medicine-Medicine (miscellaneous)
CiteScore
45.30
自引率
1.10%
发文量
138
期刊介绍:
Nature Biomedical Engineering is an online-only monthly journal that was launched in January 2017. It aims to publish original research, reviews, and commentary focusing on applied biomedicine and health technology. The journal targets a diverse audience, including life scientists who are involved in developing experimental or computational systems and methods to enhance our understanding of human physiology. It also covers biomedical researchers and engineers who are engaged in designing or optimizing therapies, assays, devices, or procedures for diagnosing or treating diseases. Additionally, clinicians, who make use of research outputs to evaluate patient health or administer therapy in various clinical settings and healthcare contexts, are also part of the target audience.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


