E. Michael Lewiecki, Edward Czerwinski, Chris Recknor, Anna Strzelecka, Guillermo Valenzuela, Mary Lawrence, Stuart Silverman, Jose Cardona, Susan M. Nattrass, Neil Binkley, Miriam Annett, Leny Pearman, Bruce Mitlak
下载PDF
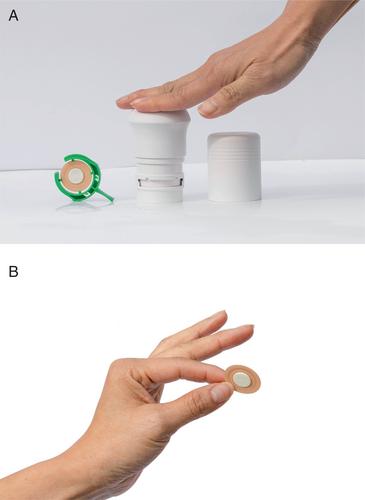
{"title":"Efficacy and Safety of Transdermal Abaloparatide in Postmenopausal Women with Osteoporosis: A Randomized Study","authors":"E. Michael Lewiecki, Edward Czerwinski, Chris Recknor, Anna Strzelecka, Guillermo Valenzuela, Mary Lawrence, Stuart Silverman, Jose Cardona, Susan M. Nattrass, Neil Binkley, Miriam Annett, Leny Pearman, Bruce Mitlak","doi":"10.1002/jbmr.4877","DOIUrl":null,"url":null,"abstract":"<p>Anabolic therapies, recommended for patients at very high fracture risk, are administered subcutaneously (SC). The objective of this study was to evaluate the efficacy and safety of the abaloparatide microstructured transdermal system (abaloparatide-sMTS) as an alternative to the SC formulation. This phase 3, noninferiority study (NCT04064411) randomly assigned postmenopausal women with osteoporosis (<i>N</i> = 511) 1:1 to open-label abaloparatide administered daily via abaloparatide-sMTS or SC injection for 12 months. The primary comparison between treatment groups was the percentage change in lumbar spine bone mineral density (BMD) at 12 months, with a noninferiority margin of 2.0%. Secondary endpoints included percentage change in total hip and femoral neck BMD, bone turnover markers, dermatologic safety, and new clinical fracture incidence. At 12 months, percentage increase from baseline in lumbar spine BMD was 7.14% (SE: 0.46%) for abaloparatide-sMTS and 10.86% (SE: 0.48%) for abaloparatide-SC (treatment difference: −3.72% [95% confidence interval: −5.01%, −2.43%]). Percentage change in total hip BMD was 1.97% for abaloparatide-sMTS and 3.70% for abaloparatide-SC. Median changes from baseline at 12 months in serum procollagen type I N-terminal propeptide (s-PINP) were 52.6% for abaloparatide-sMTS and 74.5% for abaloparatide-SC. Administration site reactions were the most frequently reported adverse events (abaloparatide-sMTS, 94.4%; abaloparatide-SC, 70.5%). Incidence of serious adverse events was similar between groups. Mild or moderate skin reactions occurred with abaloparatide-sMTS with no identifiable risk factors for sensitization reactions. Few new clinical fractures occurred in either group. Noninferiority of abaloparatide-sMTS to abaloparatide-SC for percentage change in spine BMD at 12 months was not demonstrated; however, clinically meaningful increases from baseline in lumbar spine and total hip BMD were observed in both treatment groups. © 2023 Radius Health, Inc and The Authors. <i>Journal of Bone and Mineral Research</i> published by Wiley Periodicals LLC on behalf of American Society for Bone and Mineral Research (ASBMR).</p>","PeriodicalId":185,"journal":{"name":"Journal of Bone and Mineral Research","volume":"38 10","pages":"1404-1414"},"PeriodicalIF":5.9000,"publicationDate":"2023-07-07","publicationTypes":"Journal Article","fieldsOfStudy":null,"isOpenAccess":false,"openAccessPdf":"https://onlinelibrary.wiley.com/doi/epdf/10.1002/jbmr.4877","citationCount":"1","resultStr":null,"platform":"Semanticscholar","paperid":null,"PeriodicalName":"Journal of Bone and Mineral Research","FirstCategoryId":"3","ListUrlMain":"https://onlinelibrary.wiley.com/doi/10.1002/jbmr.4877","RegionNum":1,"RegionCategory":"医学","ArticlePicture":[],"TitleCN":null,"AbstractTextCN":null,"PMCID":null,"EPubDate":"","PubModel":"","JCR":"Q1","JCRName":"ENDOCRINOLOGY & METABOLISM","Score":null,"Total":0}
引用次数: 1
引用
批量引用
Abstract
Anabolic therapies, recommended for patients at very high fracture risk, are administered subcutaneously (SC). The objective of this study was to evaluate the efficacy and safety of the abaloparatide microstructured transdermal system (abaloparatide-sMTS) as an alternative to the SC formulation. This phase 3, noninferiority study (NCT04064411) randomly assigned postmenopausal women with osteoporosis (N = 511) 1:1 to open-label abaloparatide administered daily via abaloparatide-sMTS or SC injection for 12 months. The primary comparison between treatment groups was the percentage change in lumbar spine bone mineral density (BMD) at 12 months, with a noninferiority margin of 2.0%. Secondary endpoints included percentage change in total hip and femoral neck BMD, bone turnover markers, dermatologic safety, and new clinical fracture incidence. At 12 months, percentage increase from baseline in lumbar spine BMD was 7.14% (SE: 0.46%) for abaloparatide-sMTS and 10.86% (SE: 0.48%) for abaloparatide-SC (treatment difference: −3.72% [95% confidence interval: −5.01%, −2.43%]). Percentage change in total hip BMD was 1.97% for abaloparatide-sMTS and 3.70% for abaloparatide-SC. Median changes from baseline at 12 months in serum procollagen type I N-terminal propeptide (s-PINP) were 52.6% for abaloparatide-sMTS and 74.5% for abaloparatide-SC. Administration site reactions were the most frequently reported adverse events (abaloparatide-sMTS, 94.4%; abaloparatide-SC, 70.5%). Incidence of serious adverse events was similar between groups. Mild or moderate skin reactions occurred with abaloparatide-sMTS with no identifiable risk factors for sensitization reactions. Few new clinical fractures occurred in either group. Noninferiority of abaloparatide-sMTS to abaloparatide-SC for percentage change in spine BMD at 12 months was not demonstrated; however, clinically meaningful increases from baseline in lumbar spine and total hip BMD were observed in both treatment groups. © 2023 Radius Health, Inc and The Authors. Journal of Bone and Mineral Research published by Wiley Periodicals LLC on behalf of American Society for Bone and Mineral Research (ASBMR).
经皮阿巴洛帕肽治疗绝经后骨质疏松症的疗效和安全性:一项随机研究
推荐用于骨折风险极高的患者的合成代谢疗法是皮下给药(SC)。本研究的目的是评估作为SC制剂替代品的阿巴洛肽微结构透皮系统(阿巴洛肽sMTS)的有效性和安全性。这项3期非劣效性研究(NCT04064411)随机分配绝经后骨质疏松症妇女(N = 511)通过阿巴洛肽sMTS或SC注射每天给予1∶1至开放标签阿巴洛肽12 月。治疗组之间的主要比较是12岁时腰椎骨密度(BMD)的百分比变化 次要终点包括髋关节和股骨颈总骨密度的百分比变化、骨转换标志物、皮肤病安全性和新的临床骨折发生率。在12 月,阿巴拉肽sMTS和阿巴拉肽SC的腰椎BMD比基线增加百分比分别为7.14%(SE:0.46%)和10.86%(SE:0.48%)(治疗差异:−3.72%[95%置信区间:−5.01%,−2.43%])。12时与基线相比的中位数变化 血清I型前胶原N-末端前肽(s-PINP)的月数分别为阿巴拉肽sMTS的52.6%和阿巴拉肽-SC的74.5%。给药部位反应是最常报告的不良事件(阿巴洛肽sMTS,94.4%;阿巴洛肽SC,70.5%)。两组之间严重不良事件的发生率相似。阿巴洛肽sMTS发生轻度或中度皮肤反应,没有可识别的致敏反应风险因素。两组均未出现新的临床骨折。阿巴洛肽sMTS对12岁时脊柱骨密度百分比变化的非劣效性 月没有得到证明;然而,在两个治疗组中都观察到腰椎和髋关节总骨密度比基线有临床意义的增加。©2023 Radius Health,Inc和作者。由Wiley Periodicals LLC代表美国骨与矿物研究学会(ASBMR)出版的《骨与矿产研究杂志》。
本文章由计算机程序翻译,如有差异,请以英文原文为准。

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


