Synthesis towards Phainanoid F: Photo-induced 6π-Electrocyclization for Constructing Contiguous All-Carbon Quaternary Centers
IF 3.5
3区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
In this paper, we report an efficient strategy for synthesizing the DEFGH rings of phainanoid F. The key to the construction of the 13,30-cyclodammarane skeleton of the molecule was a photo-induced 6π-electrocyclization and a homoallylic elimination. Notably, this is a rare example of using electrocyclization reaction to simultaneously construct two vicinal quaternary carbons in total synthesis. The strategy outlined here forms the basis of our total synthesis of Phainanoid F, and it could also serve as a generally applicable approach for synthesizing other natural products containing similar 13,30-cyclodammarane skeletons.
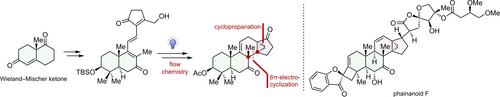
类Phainanoid F的合成:光诱导6π-电循环构建连续的全碳四元中心
在本文中,我们报道了一种合成类phainanoid F的DEFGH环的有效策略。构建该分子的13,30环达曼烷骨架的关键是光诱导的6π-电环化和高烯丙基消除。值得注意的是,这是在全合成中使用电环化反应同时构建两个相邻的季碳的罕见例子。本文概述的策略构成了我们全面合成Phainanoid F的基础,它也可以作为合成其他含有类似13,30环达曼烷骨架的天然产物的通用方法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemistry - An Asian Journal
化学-化学综合
CiteScore
7.00
自引率
2.40%
发文量
535
审稿时长
1.3 months
期刊介绍:
Chemistry—An Asian Journal is an international high-impact journal for chemistry in its broadest sense. The journal covers all aspects of chemistry from biochemistry through organic and inorganic chemistry to physical chemistry, including interdisciplinary topics.
Chemistry—An Asian Journal publishes Full Papers, Communications, and Focus Reviews.
A professional editorial team headed by Dr. Theresa Kueckmann and an Editorial Board (headed by Professor Susumu Kitagawa) ensure the highest quality of the peer-review process, the contents and the production of the journal.
Chemistry—An Asian Journal is published on behalf of the Asian Chemical Editorial Society (ACES), an association of numerous Asian chemical societies, and supported by the Gesellschaft Deutscher Chemiker (GDCh, German Chemical Society), ChemPubSoc Europe, and the Federation of Asian Chemical Societies (FACS).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


