Egg provisioning explains the penetrance of symbiont-mediated sex allocation distortion in haplodiploids
IF 4.3
3区 材料科学
Q1 ENGINEERING, ELECTRICAL & ELECTRONIC
引用次数: 0
Abstract
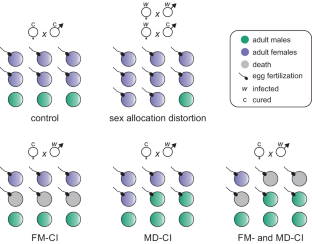
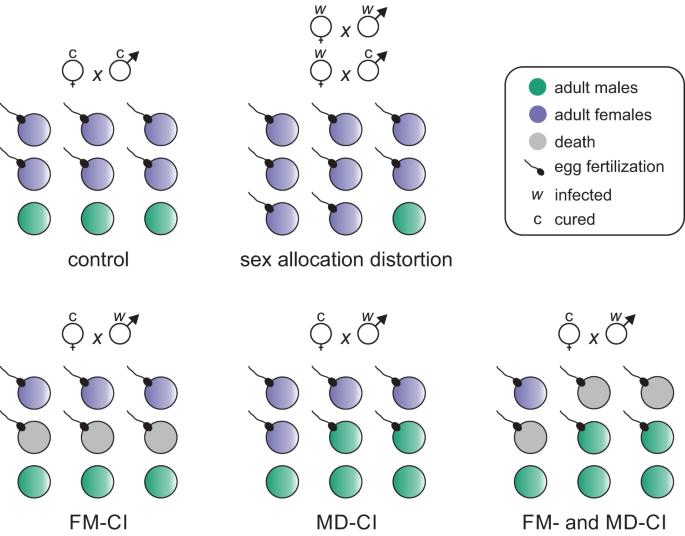
Maternally transmitted symbionts such as Wolbachia can alter sex allocation in haplodiploid arthropods. By biasing population sex ratios towards females, these changes in sex allocation may facilitate the spread of symbionts. In contrast to symbiont-induced cytoplasmic incompatibility (CI), the mechanisms that underpin sex allocation distortion remain poorly understood. Using a nuclear genotype reference panel of the haplodiploid mite Tetranychus urticae and a single Wolbachia variant that is able to simultaneously induce sex allocation distortion and CI, we unraveled the mechanistic basis of Wolbachia-mediated sex allocation distortion. Host genotype was an important determinant for the strength of sex allocation distortion. We further show that sex allocation distortion by Wolbachia in haplodiploid mites is driven by increasing egg size, hereby promoting egg fertilization. This change in reproductive physiology was also coupled to increased male and female adult size. Our results echo previous work on Cardinium symbionts, suggesting that sex allocation distortion by regulating host investment in egg size is a common strategy among symbionts that infect haplodiploids. To better understand the relevance that sex allocation distortion may have for the spread of Wolbachia in natural haplodiploid populations, we parametrized a model based on generated phenotypic data. Our simulations show that empirically derived levels of sex allocation distortion can be sufficient to remove invasion thresholds, allowing CI to drive the spread of Wolbachia independently of the initial infection frequency. Our findings help elucidate the mechanisms that underlie the widespread occurrence of symbionts in haplodiploid arthropods and the evolution of sex allocation.
卵子供给解释了单倍体中共生体介导的性别分配扭曲的穿透性
沃尔巴克氏体等经由母体传播的共生体可以改变单倍体节肢动物的性别分配。通过使种群性别比例偏向雌性,这些性别分配的变化可能会促进共生体的传播。与共生体诱导的细胞质不相容(CI)相比,人们对性别分配扭曲的机制仍然知之甚少。我们利用单倍体螨虫 Tetranychus urticae 的核基因型参考面板和能够同时诱导性别分配扭曲和 CI 的单一沃尔巴克氏体变体,揭示了沃尔巴克氏体介导的性别分配扭曲的机理基础。宿主基因型是性别分配扭曲强度的重要决定因素。我们进一步发现,在单倍体螨类中,沃尔巴克氏体的性别分配扭曲是通过增加卵的大小来驱动的,从而促进卵的受精。生殖生理的这种变化还与雌雄成体尺寸的增加有关。我们的研究结果与之前关于红心螨共生体的研究结果一致,表明通过调节宿主对卵大小的投资来扭曲性别分配是感染单倍体螨虫的共生体的共同策略。为了更好地理解性别分配扭曲可能与沃尔巴克氏体在自然单倍体种群中传播的相关性,我们根据生成的表型数据对模型进行了参数化。我们的模拟结果表明,根据经验得出的性别分配扭曲水平足以消除入侵阈值,从而使CI能够独立于初始感染频率而驱动沃尔巴克氏体的传播。我们的发现有助于阐明共生体在单倍体节肢动物中的广泛存在以及性别分配进化的机制。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


