Outcome of rare primary malignant bone sarcoma treated with multimodal therapy: Results from the EUROpean Bone Over 40 Sarcoma Study (EURO-B.O.S.S.)
Abstract
Background
Rare primary malignant bone sarcomas (RPMBS) account for 5%–10% of primary high-grade bone tumors and represent a major treatment challenge. The outcome of patients with RPMBS enrolled in the EUROpean Bone Over 40 Sarcoma Study (EURO-B.O.S.S) is presented.
Methods
Inclusion criteria were as follows: age from 41 to 65 years and a diagnosis of high-grade spindle cell, pleomorphic, or vascular RPMBS. The chemotherapy regimen included doxorubicin 60 mg/m2, ifosfamide 9 g/m2, and cisplatin 90 mg/m2; postoperative methotrexate 8 g/m2 was added in case of a poor histologic response. Version 2.0 of the Common Terminology Criteria for Adverse Events, Kaplan–Meier curves, log-rank tests, and univariate Cox regression models were used.
Results
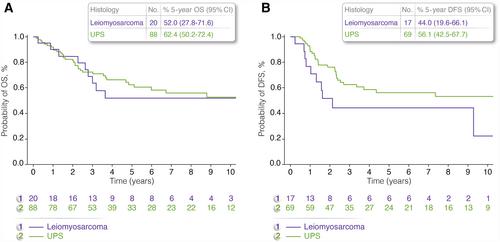
In total, 113 patients were evaluable for analysis. The median patient age was 52 years (range, 40–66 years), and 67 patients were men. Eighty-eight tumors were categorized as undifferentiated pleomorphic sarcomas (UPS), 20 were categorized as leiomyosarcomas, three were categorized as fibrosarcomas, and two were categorized as angiosarcomas. Eighty-three of 113 tumors were located in the extremities. Ninety-five of 113 patients presented with no evidence of metastases. After a median follow-up of 6.8 years (interquartile range [IQR], 3.5–9.8 years), the 5-year overall survival rate for patients with localized disease was 68.4% (IQR, 56.9%–77.5%), and it was 71.7% (IQR, 58.1%–81.6%) for patients with UPS and 54.9% (IQR, 29.5%–74.5%) for patients with leiomyosarcoma. Grade III–IV hematologic toxicity was reported in 81% patients; 23% had grade II–III neurotoxicity, and 37.5% had grade I–II nephrotoxicity. Five-year overall survival was significantly better for patients with localized disease, for patients who obtained surgical complete remission, and when the primary tumor was located in the extremities.
Conclusions
The survival of patients who had RPMBS in the current series was similar to that of age-matched patients who had high-grade osteosarcoma treated according to the same protocol. An osteosarcoma-like chemotherapy may be proposed in patients who have RPMBS.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


