Transport by circulating myeloid cells drives liposomal accumulation in inflamed synovium
IF 38.1
1区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 1
Abstract
The therapeutic potential of liposomes to deliver drugs into inflamed tissue is well documented. Liposomes are believed to largely transport drugs into inflamed joints by selective extravasation through endothelial gaps at the inflammatory sites, known as the enhanced permeation and retention effect. However, the potential of blood-circulating myeloid cells for the uptake and delivery of liposomes has been largely overlooked. Here we show that myeloid cells can transport liposomes to inflammatory sites in a collagen-induced arthritis model. It is shown that the selective depletion of the circulating myeloid cells reduces the accumulation of liposomes up to 50–60%, suggesting that myeloid-cell-mediated transport accounts for more than half of liposomal accumulation in inflamed regions. Although it is widely believed that PEGylation inhibits premature liposome clearance by the mononuclear phagocytic system, our data show that the long blood circulation times of PEGylated liposomes rather favours uptake by myeloid cells. This challenges the prevailing theory that synovial liposomal accumulation is primarily due to the enhanced permeation and retention effect and highlights the potential for other pathways of delivery in inflammatory diseases. PEGylated liposomal accumulation in inflamed regions has mainly been attributed to the enhanced permeation and retention effect. An arthritis model that chemotactically attracted myeloid cells shows that monocytes and neutrophils play an essential role in liposome delivery towards inflamed joints.
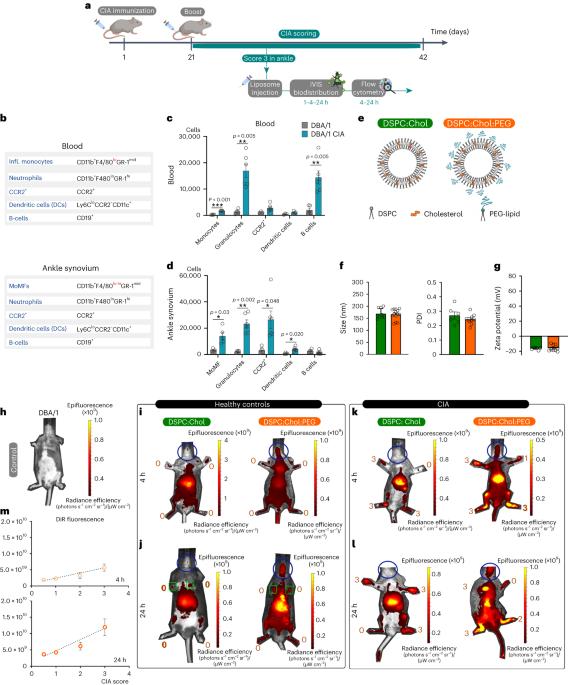
髓系细胞循环运输驱动脂质体在炎症滑膜内积聚。
脂质体将药物输送到炎症组织的治疗潜力已得到充分证明。脂质体被认为通过炎症部位的内皮间隙选择性外渗,在很大程度上将药物转运到炎症关节,称为增强渗透和滞留效应。然而,血液循环骨髓细胞摄取和输送脂质体的潜力在很大程度上被忽视了。我们在胶原诱导的关节炎模型中发现骨髓细胞可以将脂质体转运到炎症部位。研究表明,循环髓细胞的选择性耗竭可使脂质体的积累减少50-60%,这表明髓细胞介导的运输占炎症区域脂质体积累的一半以上。尽管人们普遍认为聚乙二醇化抑制单核吞噬系统对脂质体的过早清除,但我们的数据表明,聚乙二醇化脂质体的长血液循环时间更有利于髓细胞的吸收。这挑战了目前流行的理论,即滑膜脂质体积聚主要是由于增强的渗透和滞留效应,并强调了炎症性疾病中其他传递途径的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature nanotechnology
工程技术-材料科学:综合
CiteScore
59.70
自引率
0.80%
发文量
196
审稿时长
4-8 weeks
期刊介绍:
Nature Nanotechnology is a prestigious journal that publishes high-quality papers in various areas of nanoscience and nanotechnology. The journal focuses on the design, characterization, and production of structures, devices, and systems that manipulate and control materials at atomic, molecular, and macromolecular scales. It encompasses both bottom-up and top-down approaches, as well as their combinations.
Furthermore, Nature Nanotechnology fosters the exchange of ideas among researchers from diverse disciplines such as chemistry, physics, material science, biomedical research, engineering, and more. It promotes collaboration at the forefront of this multidisciplinary field. The journal covers a wide range of topics, from fundamental research in physics, chemistry, and biology, including computational work and simulations, to the development of innovative devices and technologies for various industrial sectors such as information technology, medicine, manufacturing, high-performance materials, energy, and environmental technologies. It includes coverage of organic, inorganic, and hybrid materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


