Biotin-dependent cell envelope remodelling is required for Mycobacterium abscessus survival in lung infection
IF 19.4
1区 生物学
Q1 MICROBIOLOGY
引用次数: 4
Abstract
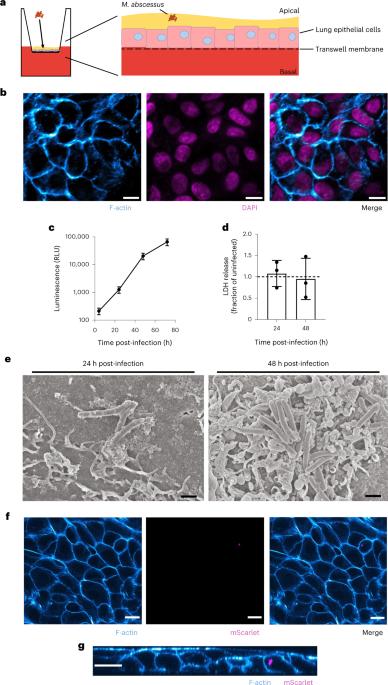
Mycobacterium abscessus is an emerging pathogen causing lung infection predominantly in patients with underlying structural abnormalities or lung disease and is resistant to most frontline antibiotics. As the pathogenic mechanisms of M. abscessus in the context of the lung are not well-understood, we developed an infection model using air–liquid interface culture and performed a transposon mutagenesis and sequencing screen to identify genes differentially required for bacterial survival in the lung. Biotin cofactor synthesis was required for M. abscessus growth due to increased intracellular biotin demand, while pharmacological inhibition of biotin synthesis prevented bacterial proliferation. Biotin was required for fatty acid remodelling, which increased cell envelope fluidity and promoted M. abscessus survival in the alkaline lung environment. Together, these results indicate that biotin-dependent fatty acid remodelling plays a critical role in pathogenic adaptation to the lung niche, suggesting that biotin synthesis and fatty acid metabolism might provide therapeutic targets for treatment of M. abscessus infection. Biotin biosynthesis enables fatty acid remodelling and increased cell envelope fluidity, which counter alkaline pH in the lung and promote Mycobacterium abscessus growth during infection.
生物素依赖的细胞包膜重塑是肺部感染脓肿分枝杆菌存活所必需的。
脓肿分枝杆菌是一种新兴病原体,主要在有潜在结构异常或肺部疾病的患者中引起肺部感染,并且对大多数一线抗生素具有耐药性。由于脓肿分枝杆菌在肺部的致病机制尚不清楚,我们利用气液界面培养建立了感染模型,并进行了转座子诱变和测序筛选,以确定肺部细菌生存所需的差异基因。由于细胞内生物素需求的增加,脓肿分枝杆菌的生长需要生物素辅助因子的合成,而生物素合成的药物抑制可以阻止细菌的增殖。生物素是脂肪酸重塑所必需的,这增加了细胞包膜的流动性,促进了脓肿分枝杆菌在碱性肺环境中的存活。综上所述,这些结果表明生物素依赖性脂肪酸重构在病原菌适应肺生态位过程中起着关键作用,提示生物素合成和脂肪酸代谢可能为治疗脓肿支原体感染提供治疗靶点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Microbiology
Immunology and Microbiology-Microbiology
CiteScore
44.40
自引率
1.10%
发文量
226
期刊介绍:
Nature Microbiology aims to cover a comprehensive range of topics related to microorganisms. This includes:
Evolution: The journal is interested in exploring the evolutionary aspects of microorganisms. This may include research on their genetic diversity, adaptation, and speciation over time.
Physiology and cell biology: Nature Microbiology seeks to understand the functions and characteristics of microorganisms at the cellular and physiological levels. This may involve studying their metabolism, growth patterns, and cellular processes.
Interactions: The journal focuses on the interactions microorganisms have with each other, as well as their interactions with hosts or the environment. This encompasses investigations into microbial communities, symbiotic relationships, and microbial responses to different environments.
Societal significance: Nature Microbiology recognizes the societal impact of microorganisms and welcomes studies that explore their practical applications. This may include research on microbial diseases, biotechnology, or environmental remediation.
In summary, Nature Microbiology is interested in research related to the evolution, physiology and cell biology of microorganisms, their interactions, and their societal relevance.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


