Chitooligosaccharide reconstitutes intestinal mucus layer to improve oral absorption of water-soluble drugs
Abstract
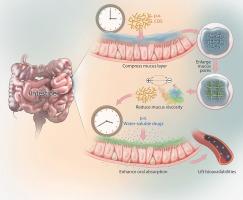
Intestinal mucus is a complex natural hydrogel barrier with unique physical properties that impede the absorption of various oral drugs. Both washout from the upper water layer and the physical resistance of the mucus layer particularly affect bioavailability of, especially, highly water-soluble molecules. One potential strategy for designing pharmaceutical formulations is to add absorption enhancers (AEs). However, there are few reports of AEs that work on mucus and their underlying mechanisms, leading to imprecise application. In this study, we investigated chitooligosaccharide (COS) as a safe, low-cost, and effective oral drug AE. We revealed the hydrodynamic law of interaction between COS and the intestinal mucus layer, which was associated with absorption benefiting mucus structural reconstruction. Based on this, we designed a translational strategy to improve the bioavailability of a group of soluble oral drugs by drinking COS solution before administration. Moreover, this research is expected to expand its application scenario by reducing drug dosage such as avoiding gastro-intestinal irritation and slowing veterinary antibiotic resistance.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


