Pathogenesis of cancers derived from thyroid follicular cells
IF 72.5
1区 医学
Q1 ONCOLOGY
引用次数: 0
Abstract
The genomic simplicity of differentiated cancers derived from thyroid follicular cells offers unique insights into how oncogenic drivers impact tumour phenotype. Essentially, the main oncoproteins in thyroid cancer activate nodes in the receptor tyrosine kinase–RAS–BRAF pathway, which constitutively induces MAPK signalling to varying degrees consistent with their specific biochemical mechanisms of action. The magnitude of the flux through the MAPK signalling pathway determines key elements of thyroid cancer biology, including differentiation state, invasive properties and the cellular composition of the tumour microenvironment. Progression of disease results from genomic lesions that drive immortalization, disrupt chromatin accessibility and cause cell cycle checkpoint dysfunction, in conjunction with a tumour microenvironment characterized by progressive immunosuppression. This Review charts the genomic trajectories of these common endocrine tumours, while connecting them to the biological states that they confer. In this Review, Fagin et al. outline the oncogenic drivers of the common endocrine tumours, which derive from thyroid follicular cells, and how these impact tumour phenotypes and disease progression.
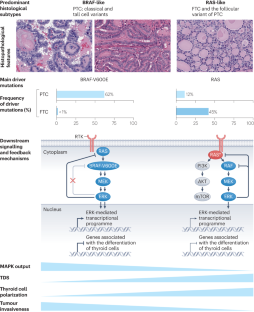
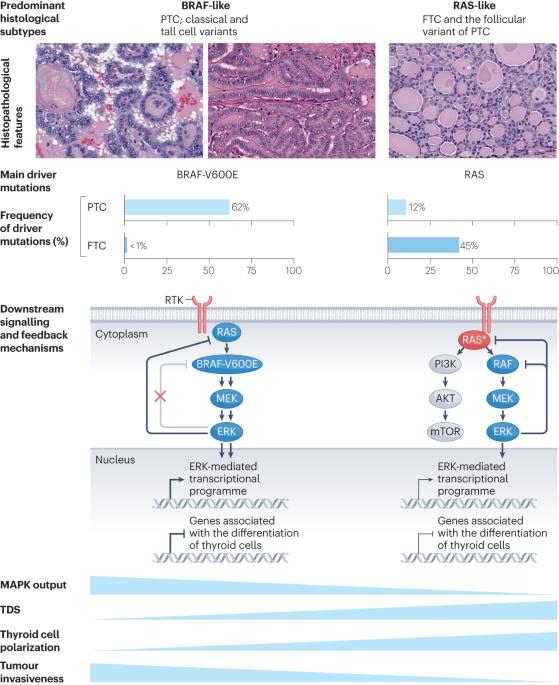
甲状腺滤泡细胞癌的发病机制。
源自甲状腺滤泡细胞的分化癌的基因组简单性为致癌驱动因素如何影响肿瘤表型提供了独特的见解。从本质上讲,癌症中的主要癌蛋白激活受体酪氨酸激酶-RAS-BRAF通路中的节点,其组成性诱导MAPK信号传导,其不同程度与其特定的生物化学作用机制一致。通过MAPK信号通路的通量大小决定了甲状腺癌症生物学的关键要素,包括分化状态、侵袭特性和肿瘤微环境的细胞组成。疾病的进展是由基因组病变引起的,这些病变驱动永生化,破坏染色质的可及性,并导致细胞周期检查点功能障碍,同时伴有以进行性免疫抑制为特征的肿瘤微环境。这篇综述描绘了这些常见内分泌肿瘤的基因组轨迹,同时将它们与它们赋予的生物学状态联系起来。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Reviews Cancer
医学-肿瘤学
CiteScore
111.90
自引率
0.40%
发文量
97
审稿时长
6-12 weeks
期刊介绍:
Nature Reviews Cancer, a part of the Nature Reviews portfolio of journals, aims to be the premier source of reviews and commentaries for the scientific communities it serves. The correct abbreviation for abstracting and indexing purposes is Nat. Rev. Cancer. The international standard serial numbers (ISSN) for Nature Reviews Cancer are 1474-175X (print) and 1474-1768 (online). Unlike other journals, Nature Reviews Cancer does not have an external editorial board. Instead, all editorial decisions are made by a team of full-time professional editors who are PhD-level scientists. The journal publishes Research Highlights, Comments, Reviews, and Perspectives relevant to cancer researchers, ensuring that the articles reach the widest possible audience due to their broad scope.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


