Suppression of beta oscillations by delayed feedback in a cortex-basal ganglia-thalamus-pedunculopontine nucleus neural loop model
Abstract
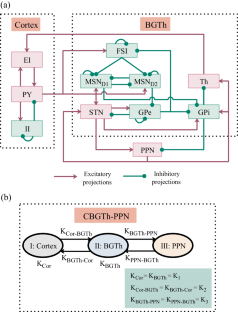
Excessive neural synchronization of neural populations in the beta (β) frequency range (12–35 Hz) is intimately related to the symptoms of hypokinesia in Parkinson’s disease (PD). Studies have shown that delayed feedback stimulation strategies can interrupt excessive neural synchronization and effectively alleviate symptoms associated with PD dyskinesia. Work on optimizing delayed feedback algorithms continues to progress, yet it remains challenging to further improve the inhibitory effect with reduced energy expenditure. Therefore, we first established a neural mass model of the cortex-basal ganglia-thalamus-pedunculopontine nucleus (CBGTh-PPN) closed-loop system, which can reflect the internal properties of cortical and basal ganglia neurons and their intrinsic connections with thalamic and pedunculopontine nucleus neurons. Second, the inhibitory effects of three delayed feedback schemes based on the external globus pallidum (GPe) on β oscillations were investigated separately and compared with those based on the subthalamic nucleus (STN) only. Our results show that all four delayed feedback schemes achieve effective suppression of pathological β oscillations when using the linear delayed feedback algorithm. The comparison revealed that the three GPe-based delayed feedback stimulation strategies were able to have a greater range of oscillation suppression with reduced energy consumption, thus improving control performance effectively, suggesting that they may be more effective for the relief of Parkinson’s motor symptoms in practical applications.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


