Bacterial Lipopolysaccharides Exacerbate Neurogenic Heterotopic Ossification Development
IF 5.1
1区 医学
Q1 ENDOCRINOLOGY & METABOLISM
Marjorie Salga, Selwin G Samuel, Hsu-Wen Tseng, Laure Gatin, Dorothée Girard, Bastien Rival, Valérie Barbier, Kavita Bisht, Svetlana Shatunova, Charlotte Debaud, Ingrid G Winkler, Julie Paquereau, Aurélien Dinh, Guillaume Genêt, Sébastien Kerever, Paer-Sélim Abback, Sébastien Banzet, François Genêt, Jean-Pierre Lévesque, Kylie A Alexander
下载PDF
{"title":"Bacterial Lipopolysaccharides Exacerbate Neurogenic Heterotopic Ossification Development","authors":"Marjorie Salga, Selwin G Samuel, Hsu-Wen Tseng, Laure Gatin, Dorothée Girard, Bastien Rival, Valérie Barbier, Kavita Bisht, Svetlana Shatunova, Charlotte Debaud, Ingrid G Winkler, Julie Paquereau, Aurélien Dinh, Guillaume Genêt, Sébastien Kerever, Paer-Sélim Abback, Sébastien Banzet, François Genêt, Jean-Pierre Lévesque, Kylie A Alexander","doi":"10.1002/jbmr.4905","DOIUrl":null,"url":null,"abstract":"<p>Neurogenic heterotopic ossifications (NHO) are heterotopic bones that develop in periarticular muscles after severe central nervous system (CNS) injuries. Several retrospective studies have shown that NHO prevalence is higher in patients who suffer concomitant infections. However, it is unclear whether these infections directly contribute to NHO development or reflect the immunodepression observed in patients with CNS injury. Using our mouse model of NHO induced by spinal cord injury (SCI) between vertebrae T<sub>11</sub> to T<sub>13</sub>, we demonstrate that lipopolysaccharides (LPS) from gram-negative bacteria exacerbate NHO development in a toll-like receptor-4 (TLR4)-dependent manner, signaling through the TIR-domain-containing adapter-inducing interferon-β (TRIF/TICAM1) adaptor rather than the myeloid differentiation primary response-88 (MYD88) adaptor. We find that T<sub>11</sub> to T<sub>13</sub> SCI did not significantly alter intestinal integrity nor cause intestinal bacteria translocation or endotoxemia, suggesting that NHO development is not driven by endotoxins from the gut in this model of SCI-induced NHO. Relevant to the human pathology, LPS increased expression of osteoblast markers in cultures of human fibro-adipogenic progenitors isolated from muscles surrounding NHO biopsies. In a case–control retrospective study in patients with traumatic brain injuries, infections with gram-negative <i>Pseudomonas</i> species were significantly associated with NHO development. Together these data suggest a functional association between gram-negative bacterial infections and NHO development and highlights infection management as a key consideration to avoid NHO development in patients. © 2023 The Authors. <i>Journal of Bone and Mineral Research</i> published by Wiley Periodicals LLC on behalf of American Society for Bone and Mineral Research (ASBMR).</p>","PeriodicalId":185,"journal":{"name":"Journal of Bone and Mineral Research","volume":"38 11","pages":"1700-1717"},"PeriodicalIF":5.1000,"publicationDate":"2023-08-21","publicationTypes":"Journal Article","fieldsOfStudy":null,"isOpenAccess":false,"openAccessPdf":"https://asbmr.onlinelibrary.wiley.com/doi/epdf/10.1002/jbmr.4905","citationCount":"0","resultStr":null,"platform":"Semanticscholar","paperid":null,"PeriodicalName":"Journal of Bone and Mineral Research","FirstCategoryId":"3","ListUrlMain":"https://onlinelibrary.wiley.com/doi/10.1002/jbmr.4905","RegionNum":1,"RegionCategory":"医学","ArticlePicture":[],"TitleCN":null,"AbstractTextCN":null,"PMCID":null,"EPubDate":"","PubModel":"","JCR":"Q1","JCRName":"ENDOCRINOLOGY & METABOLISM","Score":null,"Total":0}
引用次数: 0
引用
批量引用
Abstract
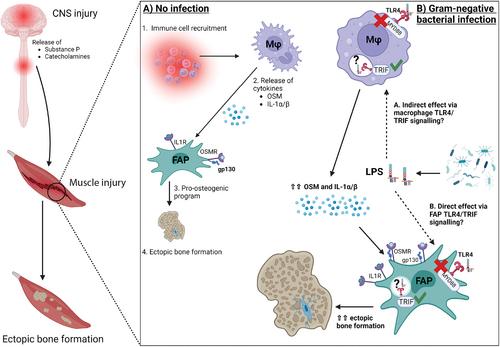
Neurogenic heterotopic ossifications (NHO) are heterotopic bones that develop in periarticular muscles after severe central nervous system (CNS) injuries. Several retrospective studies have shown that NHO prevalence is higher in patients who suffer concomitant infections. However, it is unclear whether these infections directly contribute to NHO development or reflect the immunodepression observed in patients with CNS injury. Using our mouse model of NHO induced by spinal cord injury (SCI) between vertebrae T11 to T13 , we demonstrate that lipopolysaccharides (LPS) from gram-negative bacteria exacerbate NHO development in a toll-like receptor-4 (TLR4)-dependent manner, signaling through the TIR-domain-containing adapter-inducing interferon-β (TRIF/TICAM1) adaptor rather than the myeloid differentiation primary response-88 (MYD88) adaptor. We find that T11 to T13 SCI did not significantly alter intestinal integrity nor cause intestinal bacteria translocation or endotoxemia, suggesting that NHO development is not driven by endotoxins from the gut in this model of SCI-induced NHO. Relevant to the human pathology, LPS increased expression of osteoblast markers in cultures of human fibro-adipogenic progenitors isolated from muscles surrounding NHO biopsies. In a case–control retrospective study in patients with traumatic brain injuries, infections with gram-negative Pseudomonas species were significantly associated with NHO development. Together these data suggest a functional association between gram-negative bacterial infections and NHO development and highlights infection management as a key consideration to avoid NHO development in patients. © 2023 The Authors. Journal of Bone and Mineral Research published by Wiley Periodicals LLC on behalf of American Society for Bone and Mineral Research (ASBMR).
细菌脂多糖加剧神经源性异位骨化的发展。
神经源性异位骨化(NHO)是严重中枢神经系统(CNS)损伤后在关节周围肌肉中发育的异位骨骼。几项回顾性研究表明,伴随感染的患者的NHO患病率更高。然而,尚不清楚这些感染是否直接促进了NHO的发展,或反映了在中枢神经系统损伤患者中观察到的免疫抑制。使用我们的由脊椎T11至T13之间的脊髓损伤(SCI)诱导的NHO小鼠模型,我们证明来自革兰氏阴性菌的脂多糖(LPS)以toll样受体-4(TLR4)依赖的方式加剧NHO的发育,通过含有TIR结构域的衔接子诱导干扰素-β(TRIF/TICAM1)衔接子而不是骨髓分化初级反应-88(MYD88)衔接子进行信号传导。我们发现T11至T13 SCI没有显著改变肠道完整性,也没有引起肠道细菌移位或内毒素血症,这表明在SCI诱导的NHO模型中,NHO的发展不是由肠道内毒素驱动的。与人类病理学相关,LPS增加了从NHO活检周围肌肉中分离的人类纤维脂肪祖细胞培养物中成骨细胞标志物的表达。在一项针对创伤性脑损伤患者的病例对照回顾性研究中,革兰氏阴性假单胞菌感染与NHO的发展显著相关。总之,这些数据表明革兰氏阴性细菌感染和NHO发生之间存在功能性关联,并强调感染管理是避免患者发生NHO的关键考虑因素。©2023作者。由Wiley Periodicals LLC代表美国骨与矿物研究学会(ASBMR)出版的《骨与矿产研究杂志》。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
来源期刊
期刊介绍:
The Journal of Bone and Mineral Research (JBMR) publishes highly impactful original manuscripts, reviews, and special articles on basic, translational and clinical investigations relevant to the musculoskeletal system and mineral metabolism. Specifically, the journal is interested in original research on the biology and physiology of skeletal tissues, interdisciplinary research spanning the musculoskeletal and other systems, including but not limited to immunology, hematology, energy metabolism, cancer biology, and neurology, and systems biology topics using large scale “-omics” approaches. The journal welcomes clinical research on the pathophysiology, treatment and prevention of osteoporosis and fractures, as well as sarcopenia, disorders of bone and mineral metabolism, and rare or genetically determined bone diseases.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


