Alterations of fractional anisotropy throughout cortico-basal ganglia gray matter in a macaque model of Huntington’s Disease
Abstract
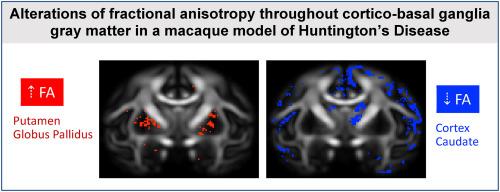
We recently generated a nonhuman primate (NHP) model of the neurodegenerative disorder Huntington's disease (HD) using adeno-associated viral vectors to express a fragment of mutant HTT protein (mHTT) throughout the cortico-basal ganglia circuit. Previous work by our group established that mHTT-treated NHPs exhibit progressive motor and cognitive phenotypes which are accompanied by mild volumetric reductions of cortical-basal ganglia structures and reduced fractional anisotropy (FA) in the white matter fiber pathways interconnecting these regions, mirroring findings observed in early-stage HD patients. Given the mild structural atrophy observed in cortical and sub-cortical gray matter regions characterized in this model using tensor-based morphometry, the current study sought to query potential microstructural alterations in the same gray matter regions using diffusion tensor imaging (DTI), to define early biomarkers of neurodegenerative processes in this model. Here, we report that mHTT-treated NHPs exhibit significant microstructural changes in several cortical and subcortical brain regions that comprise the cortico-basal ganglia circuit; with increased FA in the putamen and globus pallidus and decreased FA in the caudate nucleus and several cortical regions. DTI measures also correlated with motor and cognitive deficits such that animals with increased basal ganglia FA, and decreased cortical FA, had more severe motor and cognitive impairment. These data highlight the functional implications of microstructural changes in the cortico-basal ganglia circuit in early-stage HD.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


