家族性急性髓性白血病中的 TNFRSF13B 基因突变:复杂的遗传易感性中的新元素?
IF 3.3
4区 医学
Q2 HEMATOLOGY
引用次数: 0
摘要
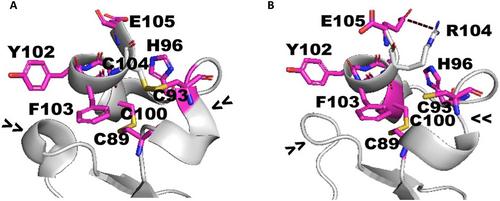
TNFRSF13B 基因突变与常见变异性免疫缺陷广泛相关。最近,TNFRSF13B 被认为是与儿童期血液恶性肿瘤发病相关的基因之一;然而,它在急性髓性白血病(AML)中的作用仍未得到探讨。我们报告了对一个家族两例急性髓性白血病病例的研究,该家族共有一个 TNFRSF13B 基因突变,该基因突变有利于与其配体 TNFSF13 形成更稳定的复合物:TNFSF13 是急性髓性白血病启动细胞的正调控因子。我们的数据使人们注意到 TNFRSF13B 在急性髓细胞性白血病发病中的作用,为骨髓性肿瘤遗传易感性的复杂情况提供了一个新的片段。本文章由计算机程序翻译,如有差异,请以英文原文为准。
TNFRSF13B gene mutation in familial acute myeloid leukemia: A new piece in the complex scenario of hereditary predisposition?
TNFRSF13B mutations are widely associated with common variable immunodeficiency. TNFRSF13B was recently counted among relevant genes associated with childhood-onset of hematological malignancies; nonetheless, its role in acute myeloid leukemia (AML) remains unexplored. We report the study of a family with two cases of AML, sharing a germline TNFRSF13B mutation favoring the formation of a more stable complex with its ligand TNFSF13: a positive regulator of AML-initiating cells. Our data turn the spotlight onto the TNFRSF13B role in AML onset, inserting a new fragment into the complex scenario of a hereditary predisposition to myeloid neoplasms.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
Hematological Oncology
医学-血液学
CiteScore
4.20
自引率
6.10%
发文量
147
审稿时长
>12 weeks
期刊介绍:
Hematological Oncology considers for publication articles dealing with experimental and clinical aspects of neoplastic diseases of the hemopoietic and lymphoid systems and relevant related matters. Translational studies applying basic science to clinical issues are particularly welcomed. Manuscripts dealing with the following areas are encouraged:
-Clinical practice and management of hematological neoplasia, including: acute and chronic leukemias, malignant lymphomas, myeloproliferative disorders
-Diagnostic investigations, including imaging and laboratory assays
-Epidemiology, pathology and pathobiology of hematological neoplasia of hematological diseases
-Therapeutic issues including Phase 1, 2 or 3 trials as well as allogeneic and autologous stem cell transplantation studies
-Aspects of the cell biology, molecular biology, molecular genetics and cytogenetics of normal or diseased hematopoeisis and lymphopoiesis, including stem cells and cytokines and other regulatory systems.
Concise, topical review material is welcomed, especially if it makes new concepts and ideas accessible to a wider community. Proposals for review material may be discussed with the Editor-in-Chief. Collections of case material and case reports will be considered only if they have broader scientific or clinical relevance.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


