二十年的激进SAM!超级家族的起源
IF 3.8
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 3
摘要
本文章由计算机程序翻译,如有差异,请以英文原文为准。
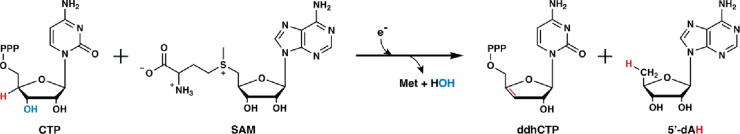
Twenty Years of Radical SAM! The Genesis of the Superfamily
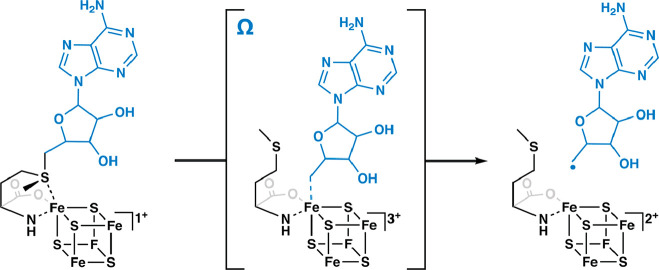
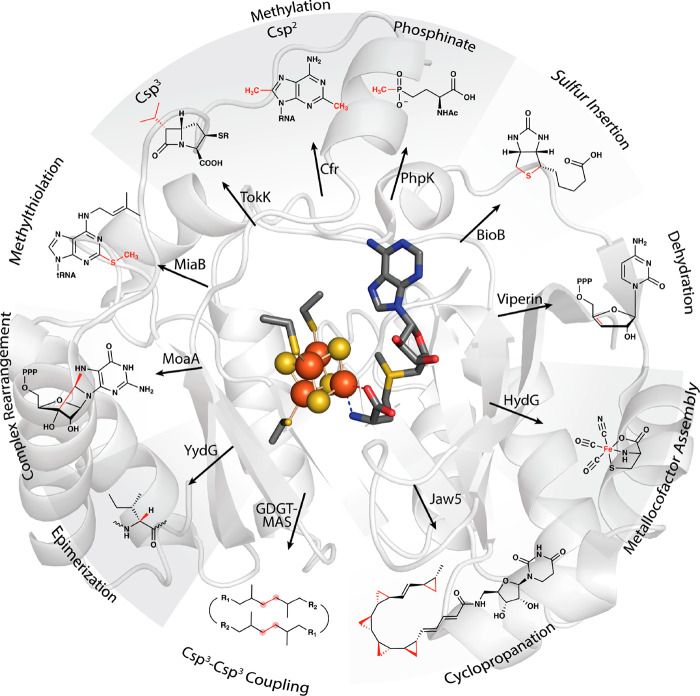
In 2001, Heidi Sofia and colleagues published a groundbreaking bioinformatics study of a superfamily of enzymes that use S-adenosylmethionine (SAM or AdoMet) to carry out a wide variety of reactions that proceed through mechanisms of catalysis involving organic radicals. This superfamily of enzymes is denoted by their ability to catalyze a reductive cleavage of SAM to methionine and a 5′-deoxyadenosyl 5′radical (5′-dA·) (Figure 1). These “Radical SAM” (RS) enzymes number over 700,000 unique sequences and catalyze over 100 distinct reactions, including the formation of stable protein radicals, complex rearrangements, methylation and thiolation of unactivated carbon centers, methylation of phosphinate phosphorus atoms, epimerization, carbon−carbon bond formation between sp2and sp3-hybridized carbon centers or two sp3-hybridized carbon centers, steps in the biosynthesis of complex metallocofactors, oxidative decarboxylation, hydroxylation, cyclopropanation, and dehydrogenation, among other reaction types (Figure 2). However, a large portion of the radical SAMeome is currently unannotated. Given the potential for a remaining reservoir of novel transformations, a major challenge is to develop strategies to annotate enzymes within the radical SAMeome. Moreover, the finding that many of these enzymes catalyze key reactions in bacteria that constitute the human microbiome, suggests the importance of the radical SAMeome in human health and disease. RS superfamily members all contain at least one [Fe4S4] cluster that is ligated by three cysteine residues (one for each of three Fe ions) that are most often found in a CxxxCxxC motif. This spacing of cysteines is conserved in at least 90% of all RS proteins and is one of the major determinants used to identify RS proteins bioinformatically. SAM associates to the fourth (unique) Fe ion in a bidentate fashion through its amino and carboxylate groups. When the cluster is reduced to the [Fe4S4] state, it induces the fragmentation of SAM to yield the 5′-dA·. In almost all RS reactions�except for the reaction catalyzed by TsrM and most likely similar reactions on analogous substrates�the role of the 5′-dA· is to abstract hydrogen atoms (H·) from a substrate, which typically initiates turnover. Studies from the Broderick and Hoffman laboratories have provided evidence for an intermediate that precedes 5′-dA· formation (Figure 1). This intermediate, termed omega, contains methionine bound to the unique iron ion of the [Fe4S4] cluster and a bond between the unique iron and the 5′-carbon of 5′-deoxyadenosine. This discovery highlights a similarity between this radical generating system and 5′-deoxyadenosyl 5′-cobalamin (AdoCbl), the other biological cofactor that is used to generate the 5′-dA·. The 5′-dA· had never been observed for many decades despite myriad attempts to do so by various investigators. In 1999, Magnusson, Reed, and Frey reported the use of S-3′,4′-anhydroadenosylmethionine, an allylic analogue of SAM, which led to the formation of a stable 5′deoxy-3′,4′-anhydroadenosyl radical in the lysine 2,3-aminomutase (KAM) reaction. This radical was then characterized extensively by electron paramagnetic resonance (EPR) spectroscopy. More recently, the groups of Broderick and Hoffman and that of Britt developed clever strategies to observe the 5′-dA· directly from SAM in the RS enzymes pyruvate formate-lyase activase (PFL-AE) and HydG. ■ THE GENESIS OF THE RADICAL SAM SUPERFAMILY
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Bio & Med Chem Au
药物、生物、化学-
CiteScore
4.10
自引率
0.00%
发文量
0
期刊介绍:
ACS Bio & Med Chem Au is a broad scope open access journal which publishes short letters comprehensive articles reviews and perspectives in all aspects of biological and medicinal chemistry. Studies providing fundamental insights or describing novel syntheses as well as clinical or other applications-based work are welcomed.This broad scope includes experimental and theoretical studies on the chemical physical mechanistic and/or structural basis of biological or cell function in all domains of life. It encompasses the fields of chemical biology synthetic biology disease biology cell biology agriculture and food natural products research nucleic acid biology neuroscience structural biology and biophysics.The journal publishes studies that pertain to a broad range of medicinal chemistry including compound design and optimization biological evaluation molecular mechanistic understanding of drug delivery and drug delivery systems imaging agents and pharmacology and translational science of both small and large bioactive molecules. Novel computational cheminformatics and structural studies for the identification (or structure-activity relationship analysis) of bioactive molecules ligands and their targets are also welcome. The journal will consider computational studies applying established computational methods but only in combination with novel and original experimental data (e.g. in cases where new compounds have been designed and tested).Also included in the scope of the journal are articles relating to infectious diseases research on pathogens host-pathogen interactions therapeutics diagnostics vaccines drug-delivery systems and other biomedical technology development pertaining to infectious diseases.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


