类器官作为个性化医疗中可靠的癌症模型,是否适用于头颈部鳞状细胞癌的精准医疗?
IF 2.9
3区 医学
Q2 GENETICS & HEREDITY
引用次数: 0
摘要
头颈部鳞状细胞癌(HNSCC)是全球第六大常见癌症。检测预测性生物标志物可改善早期诊断和预后。最近的癌症研究为癌症建模提供了一个新的途径,即在培养皿中使用被称为 "微型器官 "的器官组织,如患者衍生器官组织(PDOs)。HNSCC的负担、异质性、突变和器官组织为根据独特的基因图谱特征评估药物敏感性/耐药性反应提供了机会。作为一种高效的基因组工程技术,CRISPR(Clustered Regularly Interspaced Short Palindromic Repeat)核酸酶可用于三维(3D)器官组织中的基因操作,通过靶向致癌基因/抑癌基因建立癌症模型。此外,循环肿瘤细胞(CTCs)的单细胞分析提高了对分子血管生成、远距离转移和药物筛选的认识,而无需进行组织活检。有机体使我们能够根据患者的具体基因特征,在活体生物库中研究癌症的生物发病机制、肿瘤细胞行为和药物筛选。本文章由计算机程序翻译,如有差异,请以英文原文为准。
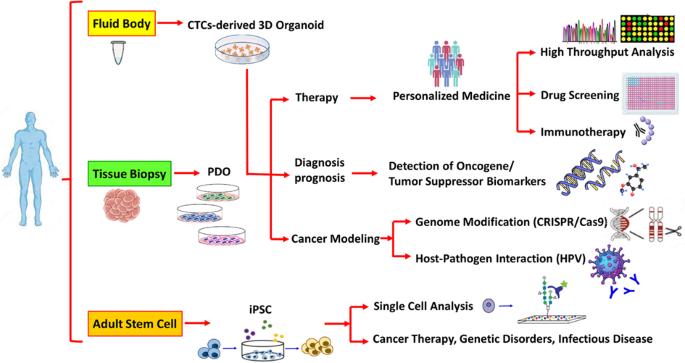
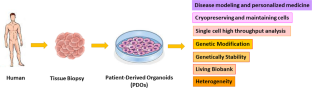
The organoid as reliable cancer modeling in personalized medicine, does applicable in precision medicine of head and neck squamous cell carcinoma?
Head and neck squamous cell carcinomas (HNSCCs) are introduced as the sixth most common cancer in the world. Detection of predictive biomarkers improve early diagnosis and prognosis. Recent cancer researches provide a new avenue for organoids, known as “mini-organs” in a dish, such as patient-derived organoids (PDOs), for cancer modeling. HNSCC burden, heterogeneity, mutations, and organoid give opportunities for the evaluation of drug sensitivity/resistance response according to the unique genetic profile signature. The Clustered Regularly Interspaced Short Palindromic Repeat (CRISPR) nucleases, as an efficient genome engineering technology, can be used for genetic manipulation in three-dimensional (3D) organoids for cancer modeling by targeting oncogenes/tumor suppressor genes. Moreover, single-cell analysis of circulating tumor cells (CTCs) improved understanding of molecular angiogenesis, distance metastasis, and drug screening without the need for tissue biopsy. Organoids allow us to investigate the biopathogenesis of cancer, tumor cell behavior, and drug screening in a living biobank according to the specific genetic profile of patients.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Pharmacogenomics Journal
医学-药学
CiteScore
7.20
自引率
0.00%
发文量
35
审稿时长
6-12 weeks
期刊介绍:
The Pharmacogenomics Journal is a print and electronic journal, which is dedicated to the rapid publication of original research on pharmacogenomics and its clinical applications.
Key areas of coverage include:
Personalized medicine
Effects of genetic variability on drug toxicity and efficacy
Identification and functional characterization of polymorphisms relevant to drug action
Pharmacodynamic and pharmacokinetic variations and drug efficacy
Integration of new developments in the genome project and proteomics into clinical medicine, pharmacology, and therapeutics
Clinical applications of genomic science
Identification of novel genomic targets for drug development
Potential benefits of pharmacogenomics.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


