Ar ' gegear ' (Ar ' = C6H3-2,6-Dipp2, Dipp = C6H3-2,6-iPr2)对炔烃的反应性:稳定二聚环丁二烯的分离
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 82
摘要
报道了“双双胺”Ar ' gegear (1, Ar ' = C6H3-2,6-Dipp2, Dipp = C6H3-2,6-iPr2)与炔烃的首次反应。1与1等量的H5C6CCC6H5反应生成高产的1,2-二聚环丁二烯2,而与2等量的阻碍较小的炔Me3SiCCH反应生成意想不到的双环化合物3。用x射线晶体学测定了2和3的分子结构。讨论了3的可能形成机理。即使在室温下,1的高反应性也强调了GeGe和CC多键之间的根本区别。本文章由计算机程序翻译,如有差异,请以英文原文为准。
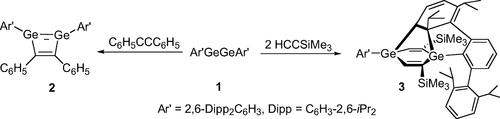
Reactivity of Ar‘GeGeAr‘ (Ar‘ = C6H3-2,6-Dipp2, Dipp = C6H3-2,6-iPr2) toward Alkynes: Isolation of a Stable Digermacyclobutadiene
The first reactions of the “digermyne” Ar‘GeGeAr‘ (1, Ar‘ = C6H3-2,6-Dipp2, Dipp = C6H3-2,6-iPr2) with alkynes are reported. 1 reacts with 1 equiv of H5C6CCC6H5 to afford the 1,2-digermacyclobutadiene 2 in high yield, while it reacts with 2 equiv of the less hindered alkyne Me3SiCCH to yield an unexpected bicyclic compound 3. Molecular structures of 2 and 3 were determined by X-ray crystallography. A possible mechanism for the formation of 3 is discussed. The high reactivity of 1, even at room temperature, emphasizes the fundamental differences between the GeGe and CC multiple bonds.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


