人Niemann-Pick C1蛋白管腔结构域C的纯化与结构
IF 1.1
4区 生物学
Q4 BIOCHEMICAL RESEARCH METHODS
Acta crystallographica. Section F, Structural biology communications
Pub Date : 2023-02-07
DOI:10.1107/S2053230X23000705
引用次数: 1
摘要
Niemann-Pick C1蛋白(NPC1)是一种主要存在于晚期内体和溶酶体中的膜蛋白,在细胞中的胆固醇稳态中发挥重要作用。NPC1的第二个管腔结构域(NPC1-C)是埃博拉病毒和马尔堡病毒的细胞内受体。本文报道了非糖基化和糖基化NPC1-C的重组生产以及非糖基蛋白的一种新的晶体形式。晶体属于空间群P21,衍射到2.3 Å分辨率。该结构与NPC1-C的其他报道结构相似,与埃博拉病毒糖蛋白或NPC2复合物中的NPC1-C相比,在突出环中观察到差异。本文章由计算机程序翻译,如有差异,请以英文原文为准。
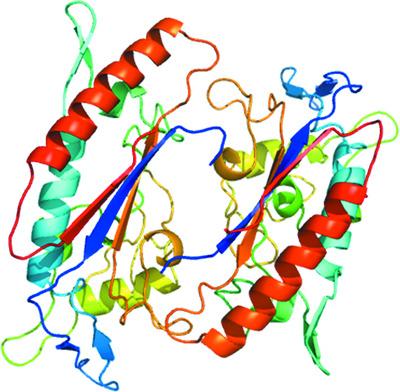
Purification and structure of luminal domain C of human Niemann–Pick C1 protein
Niemann–Pick C1 protein (NPC1) is a membrane protein that primarily resides in late endosomes and lysosomes, and plays an important role in cholesterol homeostasis in the cell. The second luminal domain of NPC1 (NPC1-C) serves as the intracellular receptor for Ebola and Marburg viruses. Here, the recombinant production of nonglycosylated and glycosylated NPC1-C and a new crystal form of the nonglycosylated protein are reported. The crystals belonged to space group P21 and diffracted to 2.3 Å resolution. The structure is similar to other reported structures of NPC1-C, with differences observed in the protruding loops when compared with NPC1-C in complex with Ebola virus glycoprotein or NPC2.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Acta crystallographica. Section F, Structural biology communications
BIOCHEMICAL RESEARCH METHODSBIOCHEMISTRY &-BIOCHEMISTRY & MOLECULAR BIOLOGY
CiteScore
1.90
自引率
0.00%
发文量
95
期刊介绍:
Acta Crystallographica Section F is a rapid structural biology communications journal.
Articles on any aspect of structural biology, including structures determined using high-throughput methods or from iterative studies such as those used in the pharmaceutical industry, are welcomed by the journal.
The journal offers the option of open access, and all communications benefit from unlimited free use of colour illustrations and no page charges. Authors are encouraged to submit multimedia content for publication with their articles.
Acta Cryst. F has a dedicated online tool called publBio that is designed to make the preparation and submission of articles easier for authors.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


