治疗转移性激素敏感前列腺癌的酶酰胺对意大利卫生预算的影响分析。
IF 0.5
Q4 HEALTH CARE SCIENCES & SERVICES
Global & Regional Health Technology Assessment
Pub Date : 2023-04-11
eCollection Date: 2023-01-01
DOI:10.33393/grhta.2023.2507
引用次数: 0
摘要
本文章由计算机程序翻译,如有差异,请以英文原文为准。
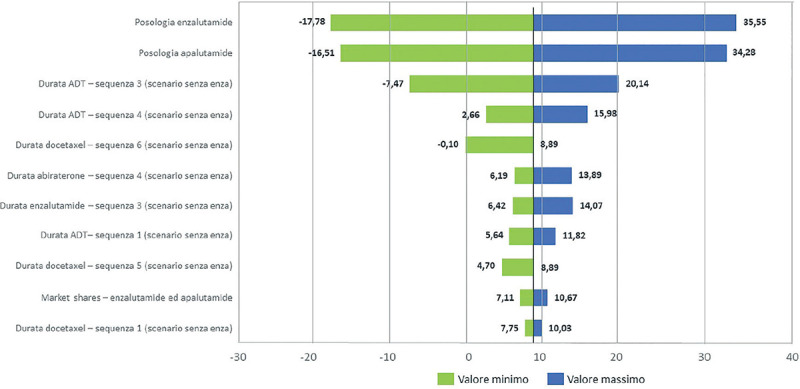
Analisi di impatto sul budget sanitario italiano di enzalutamide per il trattamento del carcinoma prostatico metastatico ormono-sensibile.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Global & Regional Health Technology Assessment
HEALTH CARE SCIENCES & SERVICES-
CiteScore
0.80
自引率
20.00%
发文量
27
审稿时长
8 weeks
期刊介绍:
Global & Regional Health Technology Assessment (GRHTA) is a peer-reviewed, open access journal which aims to promote health technology assessment and economic evaluation, enabling choices among alternative therapeutical paths or procedures with different clinical and economic outcomes. GRHTA is a unique journal having three different editorial boards who focus on their respective geographical expertise.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


