原子镧系掺杂诱导拉伸应变CuOx催化剂提高co2 - c2 +产物电还原效率
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 13
摘要
Cu是CO2还原反应(CO2RR)制备高值C2+产物的一种很有前途的电催化剂。但Cu+作为重要的C-C偶联活性位点,在还原条件下往往不稳定。原子掺杂剂如何影响铜基催化剂的性能是一个值得研究的问题。在此,我们首先计算了CO2RR与析氢反应的热力学极限势之差,以及掺杂不同金属的Cu2O上的*CO结合能,结果表明,在Cu2O中掺杂原子Gd可以有效提高催化剂的性能。在理论研究的基础上,设计了Gd1/CuOx催化剂。Gd独特的电子结构和大的离子半径不仅使Cu+在反应过程中保持稳定,还能在Gd1/CuOx中诱发拉伸应变,从而使催化剂具有良好的电还原CO2制C2+产物的性能。在−0.8 V vs可逆氢电极下,C2+产物的分电流密度为444.3 mA cm-2时,其法拉第效率可达81.4%。详细的实验和理论研究表明,Gd掺杂增强了催化剂上CO2的活化,稳定了关键中间体O*CCO,降低了C-C偶联反应的能垒。本文章由计算机程序翻译,如有差异,请以英文原文为准。
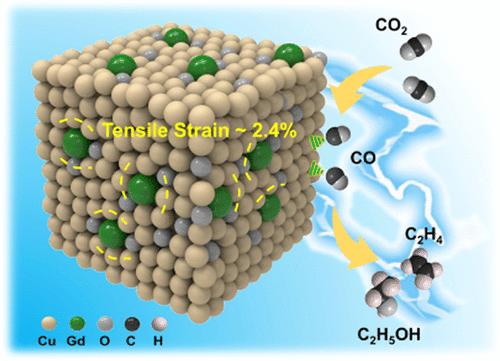
Improving CO2-to-C2+ Product Electroreduction Efficiency via Atomic Lanthanide Dopant-Induced Tensile-Strained CuOx Catalysts
Cu is a promising electrocatalyst in CO2 reduction reaction (CO2RR) to high-value C2+ products. However, as important C-C coupling active sites, the Cu+ species is usually unstable under reduction conditions. How atomic dopants affect the performance of Cu-based catalysts is interesting to be studied. Herein, we first calculated the difference between the thermodynamic limiting potentials of CO2RR and the hydrogen evolution reaction, as well as the *CO binding energy over Cu2O doped with different metals, and the results indicated that doping atomic Gd into Cu2O could improve the performance of the catalyst effectively. On the basis of the theoretical study, we designed Gd1/CuOx catalysts. The distinctive electronic structure and large ion radii of Gd not only keep the Cu+ species stable during the reaction but also induce tensile strain in Gd1/CuOx, resulting in excellent performance of the catalysts for electroreduction of CO2 to C2+ products. The Faradic efficiency of C2+ products could reach 81.4% with a C2+ product partial current density of 444.3 mA cm-2 at -0.8 V vs a reversible hydrogen electrode. Detailed experimental and theoretical studies revealed that Gd doping enhanced CO2 activation on the catalyst, stabilized the key intermediate O*CCO, and reduced the energy barrier of the C-C coupling reaction.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


